Introduction: A surface’s chemistry must be modified for certain applications, such as biosensors or conferring the property of low protein fouling. It is generally thought that a graft-from brush coating synthesized via a controlled technique, such as atom-transfer radical polymerisation (ATRP), will be maximally tailorable and versatile. We introduce the uncontrolled self-initiated photografting polymerisation (SIPGP) technique as a new means to functionalise a surface with functional ATRP initiators. Further, we hypothesise that by varying certain SIPGP conditions it is possible to alter the ATRP initiator density and subsequently create graft-from brush coatings of variable functionality.
Materials and Methods: For SIPGP, deoxygenated ATRP initiator (2-(2-chloropropanoyloxy)ethyl acrylate) in DMSO / water mixtures were applied to TCPS plates, sealed in an airtight bag, and exposed to UV radiation from a Fusion UV Light Hammer® 6 lamp. Samples were copiously washed and dried. For ATRP, deoxygenated poly(ethylene glycol) methyl ether methacrylate (PEG; Mn = 475), Cu(I)Cl, Cu(II)Cl, and 1, 1, 4, 7, 10, 10-hexamethylenetetramine in water was applied to ATRP initiator surfaces and reacted for 8 or 24 h. Samples were copiously washed and dried. XPS data was collected on an Axis HSi spectrometer (Kratos). L929 response to sterile pPEG coated 96-well TCPS plates in media (MEM + GlutaMAXTM-I, 10 % v/v FBS, 1% v/v NEAA, and 1% v/v Anti-Anti) was assessed by overnight incubation at 37 oC with 5 % CO2 and counting by a fluorescent assay (PrestoBlue™).
Results and Discussion: Figure 1A shows that TCPS was modified with ATRP initiator by SIPGP, and that the ATRP initiator remained functional and able to support graft-from ATRP polymerisation of pPEG. The ATRP initiator coating was relatively thick, but less than the XPS sampling depth of about 10 nm. The measured elemental composition (Cl/C = 0.09; O/C = 0.412) and high resolution C 1s component fitting (C1+C2:C3:C4 = 4.7:3.4:2) resemble theoretical values (Cl/C = 0.125; O/C = 0.5; C1+C2:C3:C4 = 3:3:2) reasonably well. The increase in intensity at ~289.5 eV is indicative of ester groups. For pPEG, characteristic high resolution C 1s spectral shape was observed. The dominant peak at ~286.7 eV is indicative of ether groups. Figure 1B shows that ATRP initiator surface density was likely controllable by increasing the CPOEA concentration and/or UV exposure. Those parameters, along with the ATRP reaction time, lead to variable reduction in L929 cell adhesion that was assumed to be due to generation of a more brush-like pPEG polymer coating.
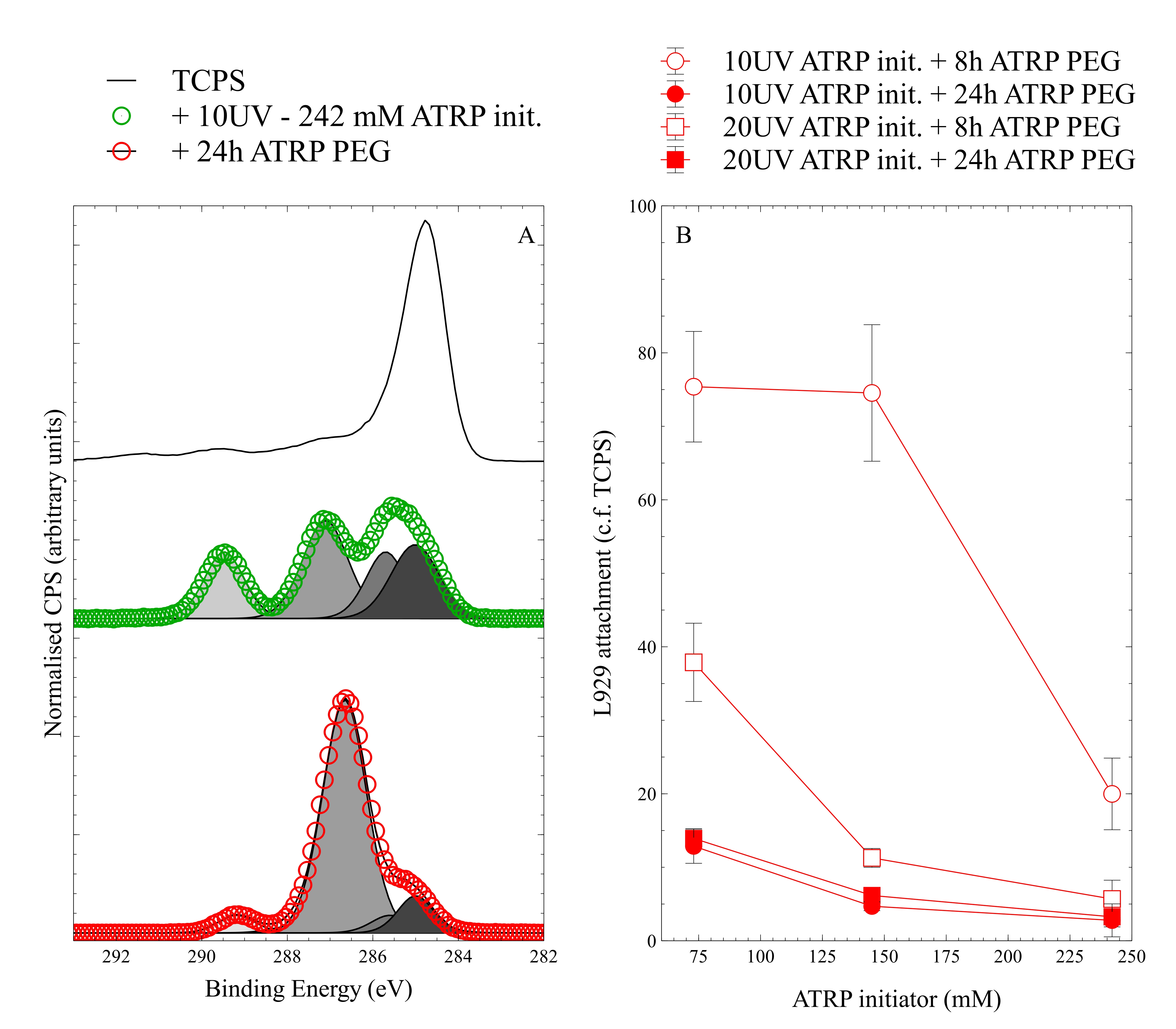
Figure 1. A) High resolution C 1s spectroscopy of TCPS substrate (black line), SIPGP graft-from ATRP initiator coating (green data), and graft-from ATRP pPEG coating (red data). Data is normalised by area, and shifted vertically for clarity. Also shown are component curve fits. B) Relative attachment of L929 mouse fibroblasts to pPEG coatings. Data is from replicate wells within a single experiment, and error bars represent ± standard error of the mean.
Conclusion: SIPGP seems a convenient means to modify a surface with ATRP initiator, and to do so in a tuneable fashion. In tuning the ATRP initiator surface density, the functionality of the resultant graft-from ATRP brush coating is also tuneable. This technique is hypothesized to work for other controlled polymerisation methods, such as RAFT, and to be useful in many bio-related fields.