Introduction: Recently, the demand for new solutions in skin regeneration has increased as there is still no "perfect" skin tissue engineering (TE) construct[1]. Here a novel gellan gum (GG) material for possible TE skin graft is proposed[2], reinforced with low concentrations of bioactive glass particles (BAG). The material has been previously studied as a possible bone scaffold[3] and has shown lower Young's modulus, suitable for soft tissue regeneration. BAG particles have been proposed as an antibacterial material[4] and enhancer of angiogenesis[5]. First the GG's gelation properties by rheology and effect of Calcium ions on it were studied, as well the effect of temperature (T) on 3D structure and degradation. Lastly, GG was in vitro evaluated.
Materials and Methods: GG hydrogels were prepared by dissolving certain weight in ultrapure water by heating it. Hydrogels formed during T drop and Ca-ions crosslinking. BAG (70 % SiO2 and 30 % CaO) was prepared via modified particulate sol-gel process. Rheology was measured by rheometer (Anton Paar Physica MCR 301).The 3D structure was observed by scanning electron microscope (SEM, Jeol JSM-7600F). For cell tests mesenchymal stem cells (MSC) isolated form animals were seeded into porous GG structures.
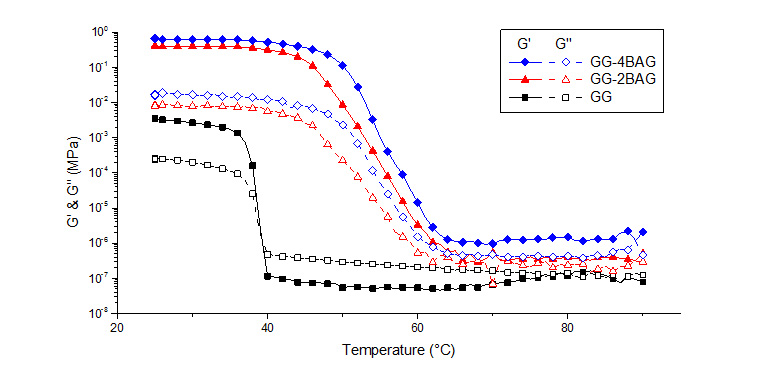
Results: In Figure 1 gelation of GG over decreasing T, with and without bioactive glass particles, is presented. With degreasing T and presence of 0.03 % Ca cross-linker the gelation T is around 40 °C, when with BAG particles the gelation T shifted to a higher T, around 60 °C.
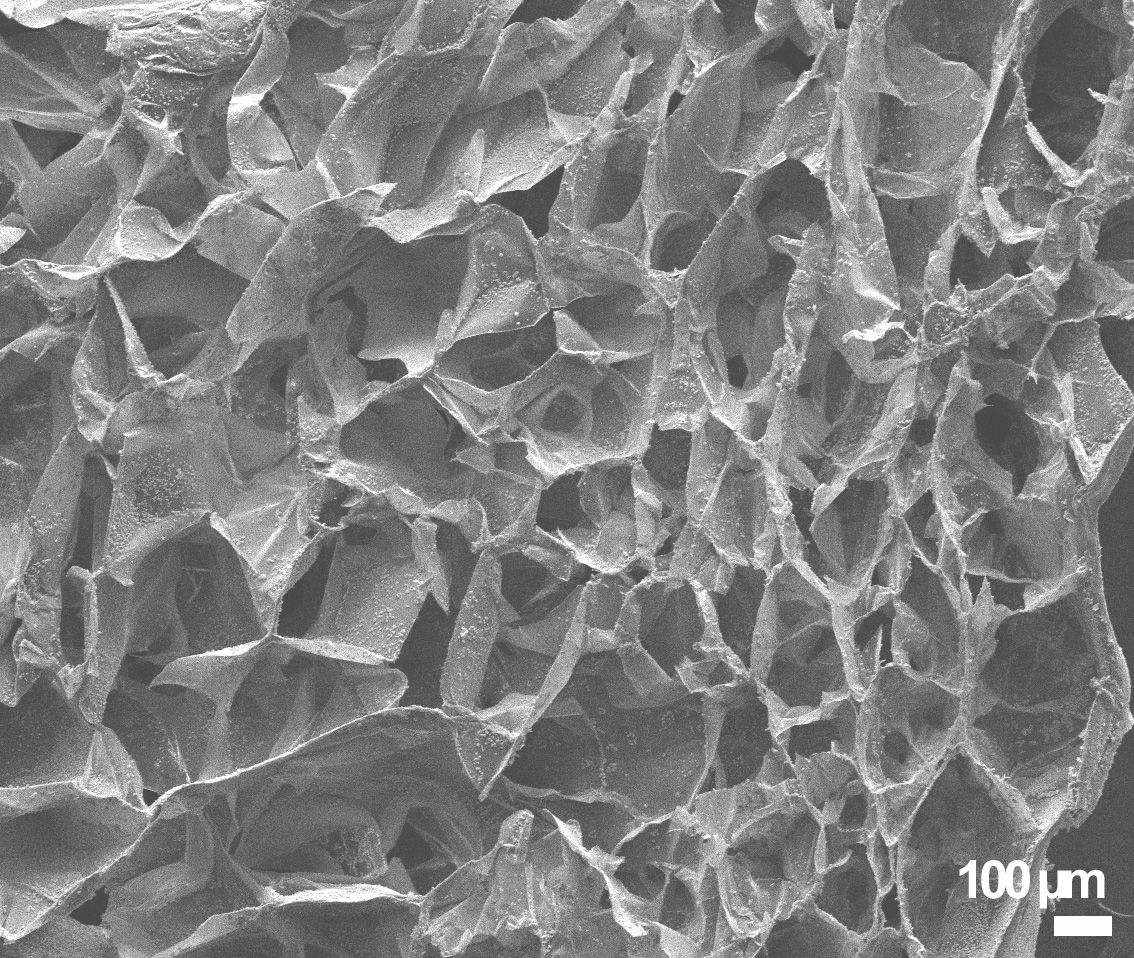
After gelation the material was freeze-dried to obtain highly porous structure. With different T (-20 °C, -80 °C and -196 °C) different pore size was observed. When freezing hydrogels to -80 °C, the pore size obtained was ~150 µm (Figure 2). When freezing hydrogels at -20 °C, pores size was estimated to over 400 µm. Roughly the same pore size was observed with -196 °C.
The degradation profile has shown high water uptake of the dried networks, which is common with the dried sponges of polysaccharides. The GG was stable over the perion of 3 weks theh it started to slowly degrade. With the GG-BAG composite the degradation occured in 7 days, due to the dissolution of the BAG prticles.
With cell testing, the porous GG structure has been evaluated as non-toxic as the cells nicely proliferated the whole GG structure.
Discussion: With the rheology measurments it was demonstrated, that GG at high T is in form of linear chains, which when cooling form double helixs that coagulate and form gel. With presence of BAG particles, the concentration of the Ca ions is increased due to dissolution of particles and caused the double helixes to aggregate faster, at high T, forming a stronger gel. The increase of Young's modulus by adding BAG particles was more than 300 %.
The porosity of the GG can be altered by freezing T. We observed that the most favorable pore size was at freezing T -80 °C. The pore size is guided by the speed of ice crystal growth and the concentration of the Ca ions, which was however equal with all freezing T.
With degradation the suitable degradation time desired was observed. The GG and GG-BAG rehydrated hydrogels degraded by hydrolysis. After 30 day the samples were still easy to handle. The sudden weight loss in GG-BAG composite was due to the dissolution of BAG particles.
With cell testing, the porous GG structure has been evaluated as non-toxic as the cells nicely proliferated the whole GG structure.
Conclusions: The GG and GG-BAG presented here have demonstrated some potential for applications in skin regeneration applications.
Luka Mohorič
References:
[1] Silva, Simone S.; et.al. Soft Constructs for Skin Tissue Engineering, in Biomimetic Approaches for Biomaterials Development . 2012.
[2] da Silva, L. P.; et. al. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta biomaterialia. Vol. 10. 2014.
[3] Gantar, A.; et. al. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Materials Science & Engineering C-Materials for Biological Applications. Vol. 43. 2014.
[4] Prabhakar, A.R.; et.al. Antibacterial effect of bioactive glass in combination with powdered enamel and dentin. Indian J Dent Res. Vol. 21. 2010.
[5] Gorustovich, A.A.; et.al. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences.Tissue Eng Part B.Vol. 16. 2010.