Introduction: Many of the strategies proposed so far to promote tissue regeneration and function recovery, based on tissue engineering principles, have failed due to the lack of vascularization of the engineered tissue. A great effort has been put into developing matrices able to support not only regeneration but also controlled angiogenesis[1].
Fistly, the aim of this work is to obtain selective cell adhesiveness on model surfaces functionalized with different percentage of two Elastin-Like-Recombinamer (ELRs) showing complementary bioactivity.
Afterwards, this two dimensional approach will provide the theoretical cues for the development of a tunable ELR- hydrogel where the QK angiogenic peptide mimicking the receptor-binding region of vascular endothelial growth factor (VEGF) is grafted chemically with controlled grafting density.
Material and Methods: The ELRs were constructed using standard genetic engineering techniques and purified using several cycles of temperature-dependent
reversible precipitation. The bioproduced polymer was purified by a series of centrifugations under and above its transition temperature. The characterization of the ELRs were accomplished by MALDI TOF mass spectroscopy, amino acid composition, FTIR, DSC and NMR techniques.
Functionalization of gold surfaces: The surfaces were cleaned with Argon plasma that effectively removes contaminations without damaging the underneath gold coating. Immersion in different recombinamer solutions for 6 hours and subsequent washing steps with milliQ water overnight were performed. The biofunctionalized surfaces were submitted to cellular adhesion assays for 48 hours with Human Foreskin Fibroblast (HFF1) and Human Umbelical Vein Endothelial Cells (HUVECs).
Hydrogel fabrication: The ELRs obtained were chemically modified at their lysine amino acids to bear the reactive groups necessary for subsequent ‘‘click chemistry’’ reaction. The QK peptide was grafted at a concentration ranging from 10nM to 100nM. The bioactivity of these matrices were further evaluated for their ability to induce the formation of tubular structure in vitro using HFF1-HUVECs co-culture system.
Results: The recombinamer composition 75% REDV and 25% RGD exhibited the best ability to enhance selective endothelial cell adhesion. It has been observed that at specific concentration QK promotes endothelial cell attachment and proliferation although at high densities it exerts an antagonistic effect.
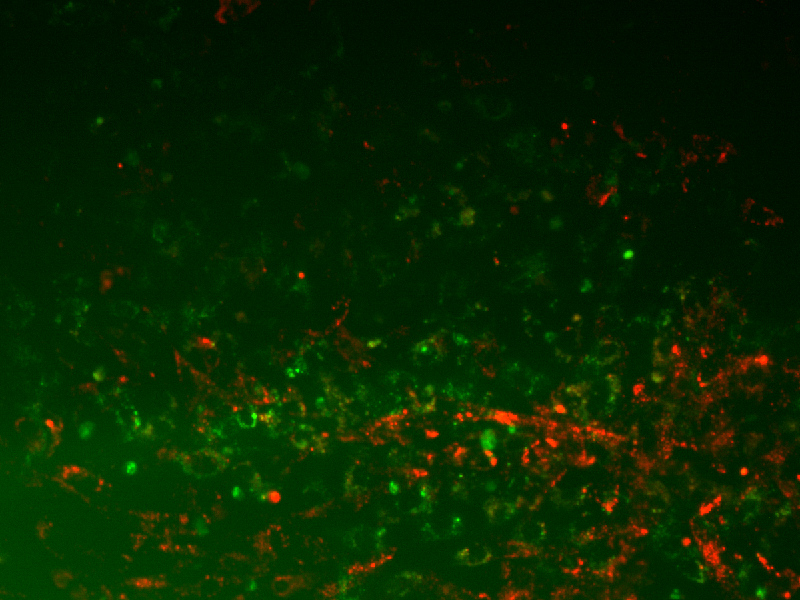
Figure1. Co-culture HUVECs and HFF1 cells adhered on a surface functionalized with 75% REDV-25% RGD ELR.
Conclusion: Our design strategy for selective cell adhesiveness on gold surfaces created a favourable environment to determine the appropriate recombinamers composition for further developing 3D matrices to be employed in reparative angiogenesis.
European Union’s FP7 grant agreement number 317304. European Union’s Horizon2020 grant agreement number 642687
References:
[1] Finetti F1, Basile A, Capasso D, Di Gaetano S, Di Stasi R, Pascale M, Turco CM, Ziche M, Morbidelli L. Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis.