Introduction: 3D printing techniques possess great potential to fabricate complex and multiscale structures for tissue engineering applications. Although many natural and synthetic polymers have been developed for 3D printing, such as poly(ethylene glycol)[1] and hyaluronic acid[2], there is still a need for more diverse properties in printed structures. Here, we synthesize and apply two photocurable elastomers from poly(glycerol-sebacate) (PGS) with acrylate and norbornene functionalities, since PGS has previously been shown to be biocompatible, degradable, and to exhibit elastomeric properties.[3]
Materials and Methods: PGS was synthesized via a polycondensation reaction[4] and used as prepolymer for further chemical modification. Acrylate-functionalized PGS (Acr-PGS) and norbornene-functionalized PGS (Nor-PGS) were prepared by the reaction of the prepolymer with acryloyl chloride and 5-norbornene-2-carbonyl chloride, respectively. Acr-PGS macromers were mixed with 0.5 wt% of the photoinitiator 2,2-dimethoxy-2-phenylacetophenone (DMPA) for acrylate polymerization (Figure 1a); Nor-PGS macromers were mixed with 0.5 wt% of DMPA and varying amounts of dithiothreitol (DTT) for thiol-norbornene click reaction (Figure 1b). Crosslinking was performed with exposure to UV light (365 nm, 10 mW/cm2). The mechanical properties of both Acr-PGS and Nor-PGS macromers were measured during polymerization using rheometry, as well as with dynamic mechanical analysis (DMA). Materials were printed by stepper-motor device extrusion from a syringe on a standard 3D printer and cured by immediate exposure to UV light (365 nm, 10 mW/cm2) upon extrusion.
Results and Discussion: Acr-PGS and Nor-PGS macromers polymerized quickly as the storage modulus (G’) increased orders of magnitude in seconds after light exposure (Figure 1a, 1b), an important criterion of photocurable materials for 3D printing. The exposure to UV light (365 nm, 10 mW/cm2, 5 mins) resulted in polymerization of Acr-PGS macromers, where the compressive moduli of Acr-PGS can be adjusted by different degrees of acrylation: ~ 20 kPa and ~ 200 kPa for 10% and 20% modification of Acr-PGS, respectively. The mechanical properties of Nor-PGS with 24% modification can be simply modulated by encapsulating different amount of DTT (~ 30 kPa and ~ 7 kPa for the norbornene-to-thiol ratio (Nor/S) = 1 and 2, respectively), allowing the use of the same functionalized Nor-PGS to present different mechanics. In particular, thiol-norbornene chemistry provides uniform network formation and low oxygen inhibition[5]. We utilized the Acr-PGS in 3D printing to fabricate a 3D structure by building up, layer by layer, a printed pattern of PGS filaments (Figure 1c).
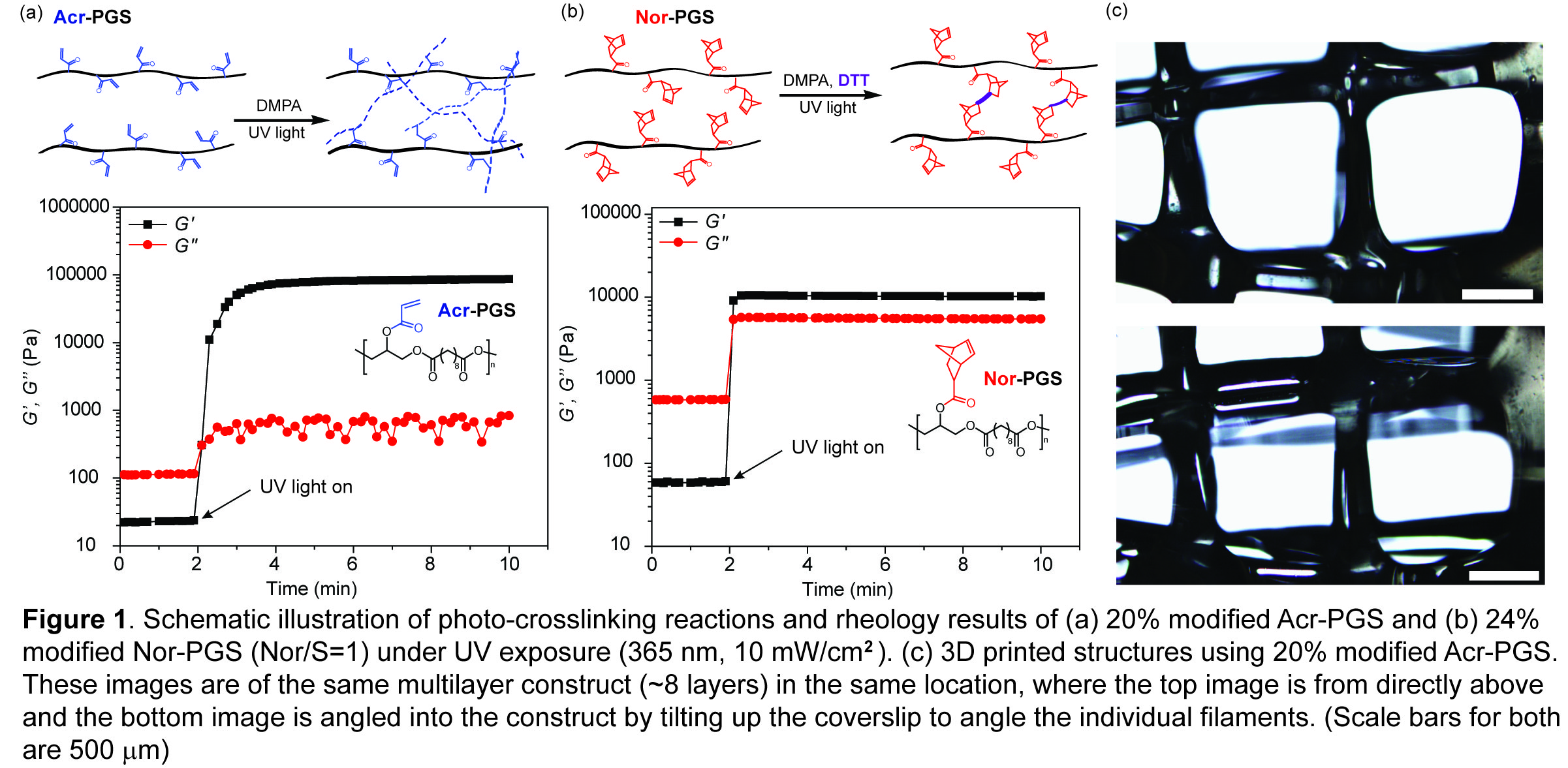
Conclusions: Here we demonstrate the functionalized PGS elastomers present fast polymerization and tunable mechanical properties, making them promising materials for 3D printing application. The mechanical properties of modified PGS materials can be fine-tuned by changing the percentage of acrylation (Acr-PGS) or adjusting the crosslinker amount (Nor-PGS). Properties of the printed structure, such as shape and material density (porosity), can be tuned in addition to the mechanical properties of the polymer itself, allowing the creation of elastomeric and biocompatible constructs for biomedical applications.
References:
[1] Rutz, A. L. et al. Adv. Mater. 2015; 27: 1607-1614.
[2] Highley, C. B. et al. Adv. Mater. 2015; 27: 5075-5079.
[3] Wang, Y. D. et al. Nat. Biotechnol. 2002; 20: 602-606.
[4] Ifkovits, J. L. et al. Biomed. Mater. 2008; 3: 034104.
[5] Hoyle, C. E. et al. Angew. Chem. Int. Ed. 2010; 49: 1540-1573.