Introduction: Anisotropic particles have been gaining popularity due to their useful properties in drug delivery applications[1]. Recently it has been shown that prolate ellipsoidal particles can serve as a platform for artificial antigen presenting cells (aAPCs)[2]. By conjugating a biomimetic Signal 1 protein and Signal 2 protein it has been demonstrated that these prolate ellipsoidal aAPCs can more effectively stimulate T-Cells than spherical counterparts. Despite these studies, it has been difficult to fully evaluate this T-Cell/particle interaction owing to the polydisperse nature of synthesized particles as well as the limited repertoire of shapes considered. The goal of this study was to develop a monodisperse, biodegradable, anisotropic particle synthesis platform for aAPC technology.
Materials and Methods: Monodisperse poly (lactic-co-glycolic acid) (PLGA) microparticles were synthesized utilizing a flow-focusing microfluidic device adapted from a previous study[3]. Poly vinyl alcohol was appropriated as the continuous phase and PLGA dissolved in dichloromethane was the focused dispersed phase. The subsequent monodisperse particles were converted to oblate ellipsoidal (1.5 fold stretch), prolate ellipsoidal (2 fold stretch), or biconvave discoid (1.5 fold stretch) particles using a thin film stretching method described previously[4]. The particles were then conjugated to MHC Db IgG gp100 dimer to serve as Signal 1 and anti CD28 to serve as Signal 2 as previously described.[2] The monodisperse aAPC were then incubated with transgenic cognate T-Cells, and proliferation was assessed with manual proliferation counting 7 days post stimulation.
Results and Discussion: Monodisperse spherical PLGA microparticles were successfully synthesized using a microfluidic flow focusing device (Figure 1a).
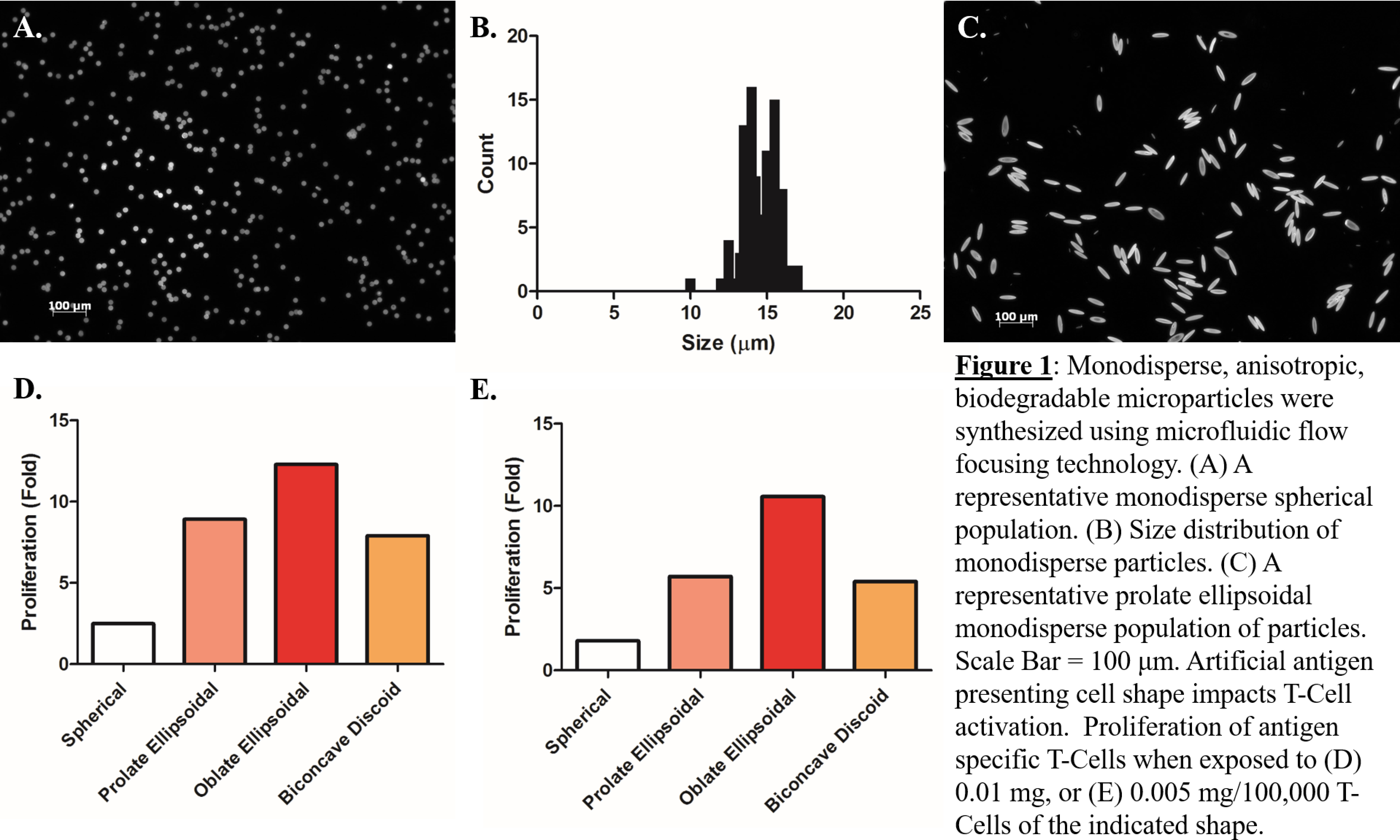
The average size of the microparticles synthesized using the method described above was 14.36 μm (Figure 1b). The polydispersity index of the microparticles was 1.05 indicating a nearly uniform population. The monodisperse spherical particles were successfully stretched into prolate ellipsoids (Figure 1c) oblate ellipsoids, and biconcave discoids. The resulting anisotropic monodisperse particles were conjugated to Signal 1 and Signal 2 proteins and coincubated with cognate T-Cells. At limiting doses, there was a 10 fold proliferative advantage of oblate ellipsoidal aAPCs over spherical aAPCs and a 2 fold proliferative advantage of oblate aAPCs over prolate aAPCs (Figure 1 d/e).
Conclusions: We have developed a platform for the synthesis of monodisperse, anisotropic, biodegradable artificial antigen presenting cells. Using flow focusing technology, we have reproducibly produced anisotropic particles of defined size and shape in a process that is amenable to scaling up for translational applications. Furthermore, we have determined that 1.5 fold stretched oblate ellipsoidal particles afford the greatest T-Cell stimulation. Investigation into the role of particle shape in the development of aAPCs will be beneficial for therapeutic application of these constructs in immune system modulation.
NIH Cancer Nanotechnology Training Center; Wallace H. Coulter Translational Partnership; NIH Grant R01-EB016721; NIH Grant P30-EY001865; Johns Hopkins Catalyst Award
References:
[1] Meyer and Green, WIREs Nanomed Nanotechnol 2015
[2] Sunshine et. al., Biomaterials 2014
[3] Xu et. al., Small 2009
[4] Meyer et. al., J Biomed Mater Res Part A 2015