Introduction: Photo-crosslinking hydrogels are widely used in tissue engineering. Their shorter crosslinking time results in higher cell viability and functionality and a more accurate structure for desired scaffolds. However, the photo-crosslinking time of hydrogels is normally tens of seconds for high-intensity light and a few minutes for low-intensity light. Thus, we present an ultrafast photo-crosslinking method for gelatin methacrylate (GelMA) hydrogel using an inexpensive laser diode. The crosslinking process is completed within ten seconds and the cell viabilities at day 0 and day 4 were approximately 90%.
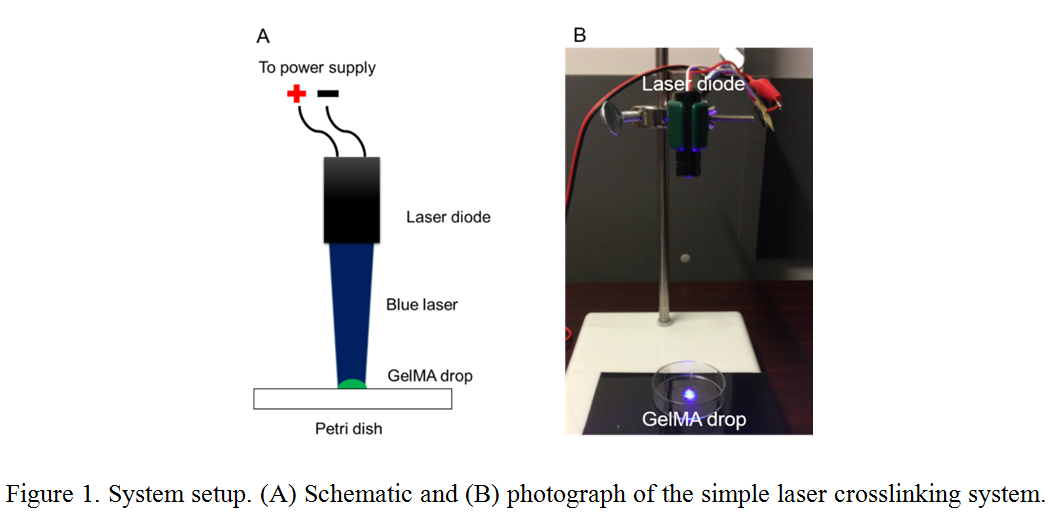
Materials and Methods: The proposed process used 10% w/v GelMA[1] and 1% w/v VA-086 photoinitiator (Wako Chem. Ltd., Osaka, Japan) dissolved in a phosphate-buffered saline (PBS). NIH 3T3 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum and 1% penicillin streptomycin (from Life Technologies, Carlsbad, CA, USA) at 37ºC and 5% CO2. A laser diode with a 405 nm wavelength and 300 mW power was placed 10 cm away from the well plate. It was powered by a DC source with 2 V and 1 A as shown in Figure 1. Before crosslinking, 8 x 106 cells/mL cells were mixed with the GelMA prepolymer solutions. Then, 1 mL of GelMA prepolymer solution with cells were added to each well and illuminated by the laser for 10 seconds. The crosslinked samples were washed three times with PBS and fresh DMEM media was added for culturing. To measure cell viability at day 0 and day 4, crosslinked samples were treated with Live/Dead cell viability assay (Biotium, Hayward, CA, USA) for 30 minutes. Cell viability was assessed by a fluorescent confocal microscope (FV1000, Olympus, Tokyo, Japan).
Results and Discussion: Figures 2A and 2B show fluorescent images of cells in the crosslinked GelMA hydrogels at day 0 and day 4. Cells were successfully encapsulated in the GelMA within 10 seconds. Figure 2C shows the result of the cell viability analysis. Cell viability was approximately 90% after 4 days of culturing. Most of the cells were attached. Thus, the developed technique is capable of crosslinking hydrogel faster than any other conventional techniques—and it is biocompatible for a variety of tissue engineering applications.

Conclusion: In this abstract, we presented a rapid photo-crosslinking technique, which can crosslink cell-laden hydrogel in ten seconds. Fluorescent images of a cell viability assay showed an approximately 90% viability 4 days after the laser-based crosslinking of the GelMA. This inexpensive crosslinking method has great potential in the rapid fabrication of micro and nano structures of hydrogels for bioprinting and tissue engineering.
This work is supported by Natural Sciences and Engineering Research Council of Canada Discovery Grant.
References:
[1] Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010;31:5536–44. doi:10.1016/j.biomaterials.2010.03.064.