Introduction: Recent reports suggest intracrine high molecular weight (hi) fibroblast growth factor 2 (FGF-2) signaling may play a role in inhibiting cell proliferation and promoting cell death[1],[2]. Herein we report on the use of alginate-graft-poly (ethylene glycol) (Alg-g-PEG) nanospheres to intracellularly deliver hi FGF-2 via a stealth mechanism, without interacting with FGF-2 cell surface receptors. We hypothesize that the intracellular delivery of FGF-2 may be a potential strategy for cancer therapy.
Materials and Methods: The preparation of Alg-g-PEG nanospheres was based on the literature by Miao et al. (2014)[3]. Briefly, Alg-g-PEG copolymers were synthesized via carbodiimide chemistry. Alg-g-PEG solution was mixed with hi FGF-2 and formed into nanospheres using a water/oil emulsion and calcium crosslinking. Carbodiimide chemistry was also performed between the Alg-g-PEG nanosphere surface and Alexa Fluor® 647 to form fluorescent nanospheres. Dynamic light scattering (DLS) was used to measure diameter and zeta-potential of FGF-2-encapsulated nanospheres. A human bFGF ELISA was used to determine FGF-2 encapsulation efficiency and in vitro release profile. For flow cytometry assays, human lung cancer cells (A549 ATTC®) were co-cultured with fluorescent Alg-g-PEG nanospheres for 24 h. For cell proliferation assays, an equal number of A549 cells were seeded into 12-well plates. Experimental groups included: cells without any treatment (control), cells with 200 µg/mL FGF-2-encapsulated nanospheres, cells with empty nanospheres, and cells with 10 ng/ml FGF-2 (extracellular). Cell viability was measured using an MTT-based assay (Sigma-Aldrich).
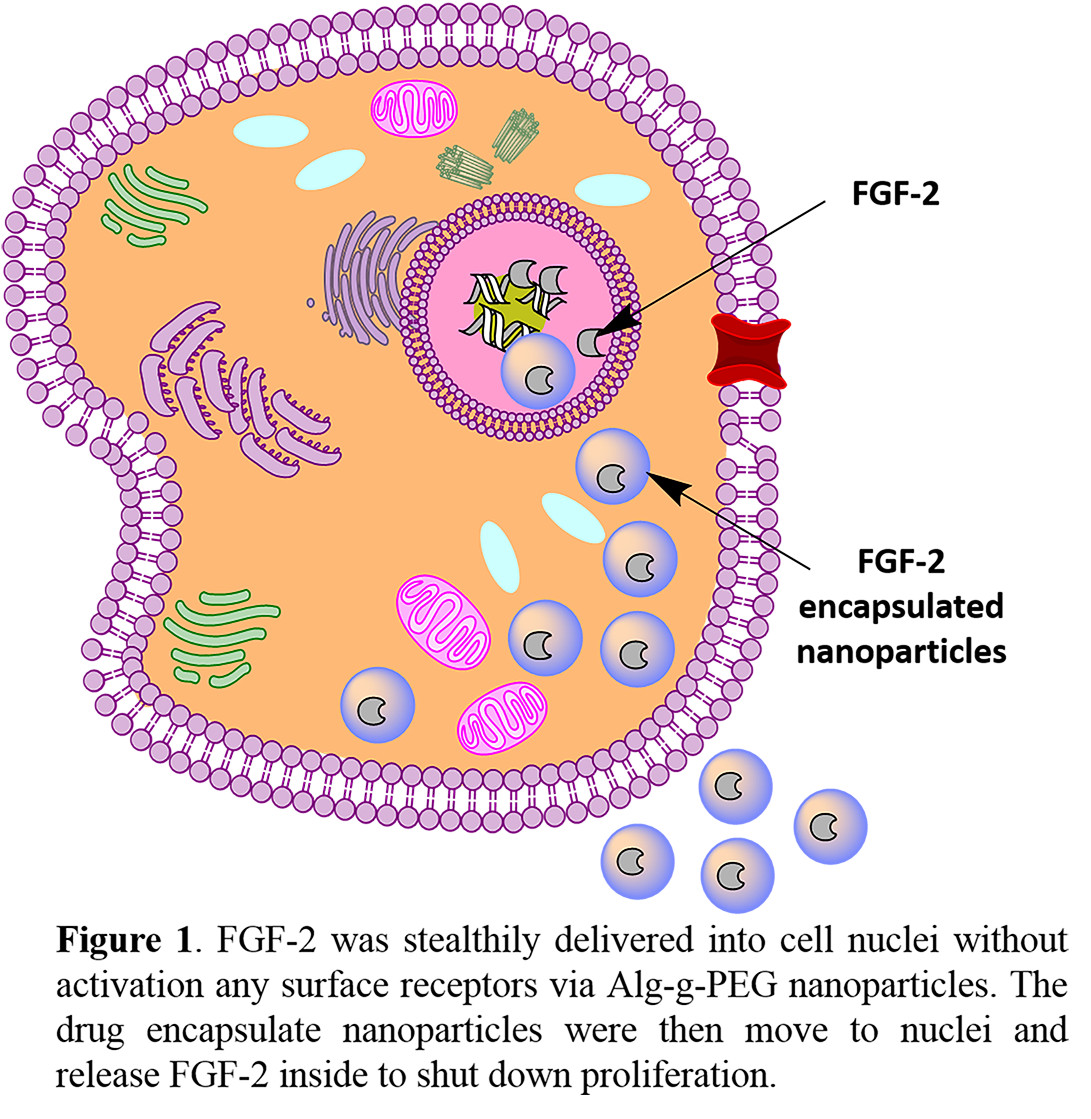
Resultsand Discussion: Figure 1 illustrates the intracellular delivery of FGF-2 into cell nuclei to shut down proliferation.
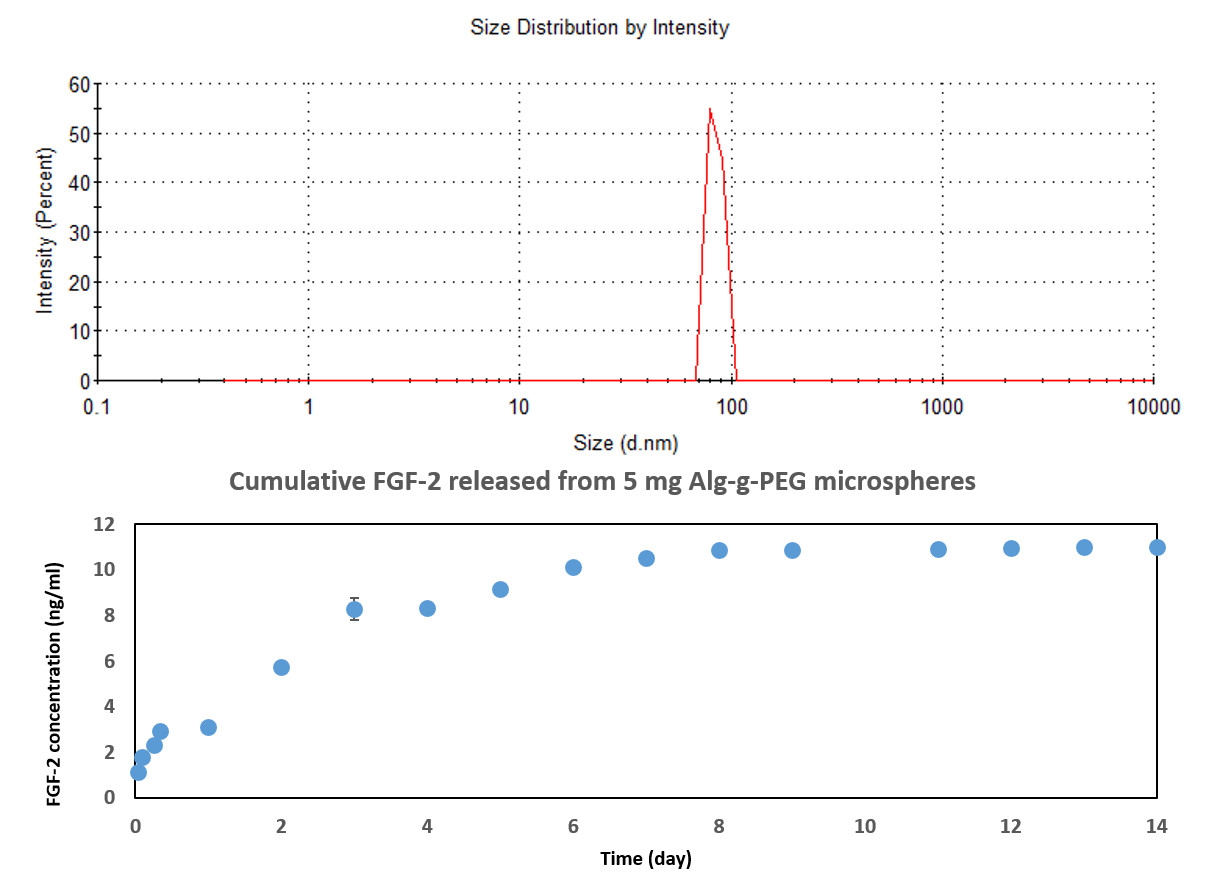
DLS determined that the nanospheres were 84 nm in diameter with a zeta-potential = -7.82 mV (see Figure 2 above). The FGF-2 release profile demonstrated a burst release within the first few days (Figure 2, bottom).
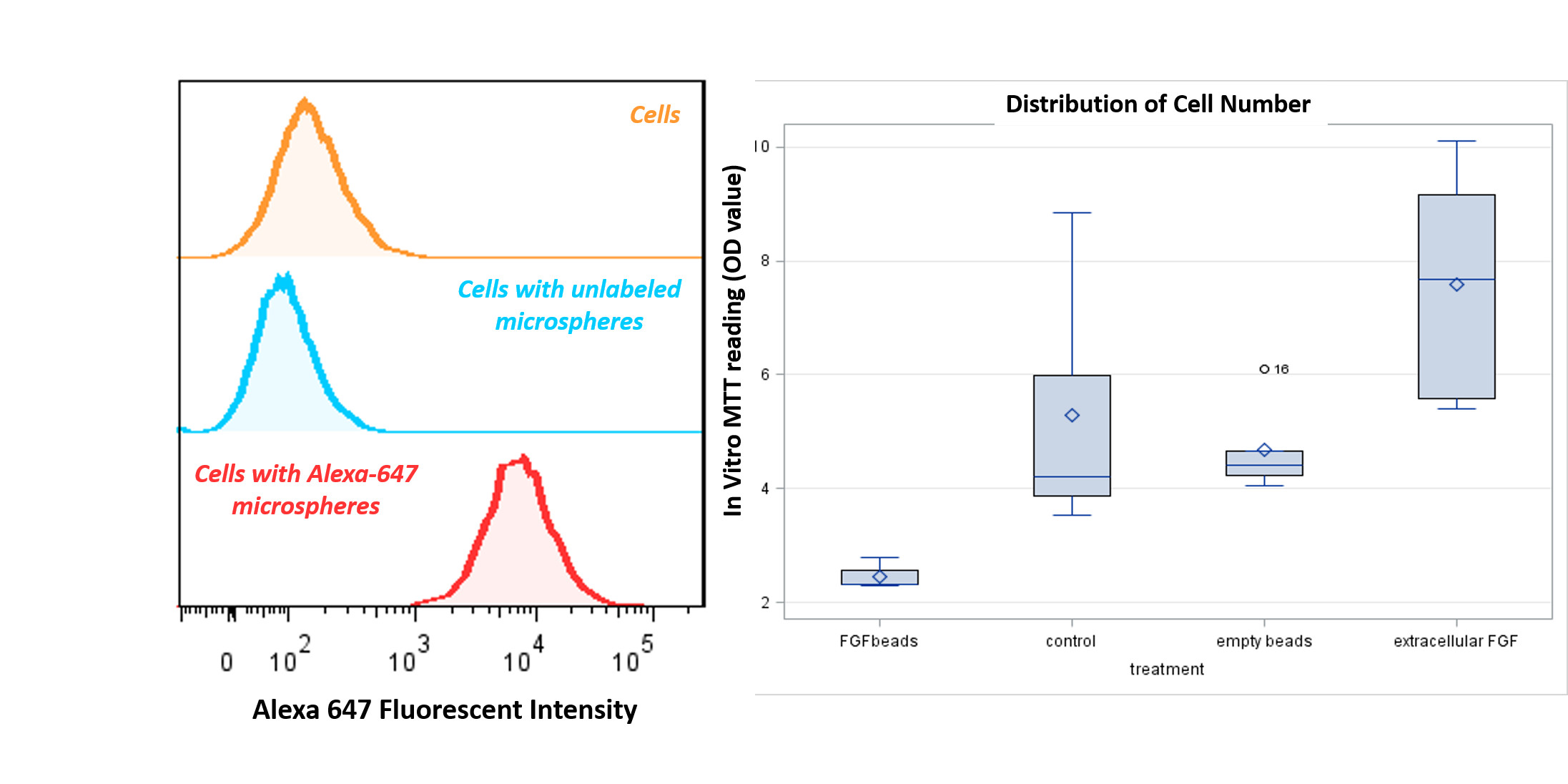
Flow cytometry showed that over 90% of the cells had internalized Alg-g-PEG nanospheres (Figure 3, Left). Cells treated with FGF-2-encapsulated nanospheres exhibited significantly lower viability (p = 0.01), whereas cells treated with extracellular FGF-2 had significantly higher viability compared with control cells (p = 0.036). The empty nanospheres had no significant effect, indicating that the Alg-g-PEG nanospheres were not cytotoxic (Figure 3, right).
Conclusion: As a potential cancer therapy, intracellular delivery of hi FGF-2 may provide an alternative treatment to chemotherapeutics or an adjunct treatment (in combination with chemotherapeutics).
The authors would love to thank Alexander Aronshtam, Ph.D. for cloning and purification of human FGF-2 and chemdraw software to complete the illustration figure
References:
[1] Ma, X.; Dang, X.; Claus, P.; Hirst, C.; Fandrich, R. R.; Jin, Y.; Grothe, C.; Kirshenbaum, L. A.; Cattini, P. A.; Kardami, E., Chromatin compaction and cell death by high molecular weight FGF-2 depend on its nuclear localization, intracrine ERK activation, and engagement of mitochondria. J Cell Physiol 2007, 213, (3), 690-8.
[2] Stachowiak, M. K.; Birkaya, B.; Aletta, J. M.; Narla, S. T.; Benson, C. A.; Decker, B.; Stachowiak, E. K., “Nuclear FGF Receptor-1 and CREB Binding Protein: An Integrative Signaling Module”. Journal of Cellular Physiology 2015, 230, (5), 989-1002.
[3] Miao, T.; Rao, K. S.; Spees, J. L.; Oldinski, R. A., Osteogenic differentiation of human mesenchymal stem cells through alginate-graft-poly(ethylene glycol) microsphere-mediated intracellular growth factor delivery. Journal of Controlled Release 2014, 192, 57-66.