For their adaptability to tissue defect and the minimally invasive procedure, injectable hydrogels are an extremely attractive class of biomaterials for soft tissue engineering. Unfortunately, inadequate diffusion of anabolic compounds and waste products frequently impairs cell survival in material core and hinders regeneration process[1]. A possible strategy to reduce limitations associated to scarce mass transport is the injection of cells loaded in rapidly degradable microcapsules. While preserving the capability to be injected and to confine cells in the desired area, the presence of spaces between the beads could favor mass transport.
Alginate, with its unique and extremely mild gelation mechanism, has a great potential for the preparation of these fast degradable microbeads, since degradation of capsules can be controlled by simply adjusting its concentration and crosslinking ion molarity[2].
Here, the possibility to tune cell release by combining alginate with macromolecules capable not only to influence capsule stability but, at the same time, to improve cell-material interaction was assessed. To this end, gelatin (porcine, type A and B) and hyaluronic acid were chosen. Microspheres were prepared, using a coaxial flow encapsulation system, by dripping in CaCl2 solution and aged in PBS at 37°C to assess swelling and weight loss and investigate the influence of components and ratios on beads stability. Mouse fibroblasts C2C12 were then encapsulated in faster degrading combinations and cell release was assessed by culturing microspheres in vitro up to four weeks. To appraise the persistence of cell release with time, microspheres were moved to new culture plates at selected time intervals and cells doubling time was taken into account to estimate actual cell release.
Among the composition tested, beads weight loss was found to be ranging from almost complete destabilization within two weeks to more than 50% capsules weight remaining at 4 weeks. Swelling rate and, above all, maximum swelling value were shown to significantly vary with composition. Formulations chosen for cell encapsulation are reported in Figure 1 and cell release profiles, for each ml of microcapsules, are shown in figure 2.
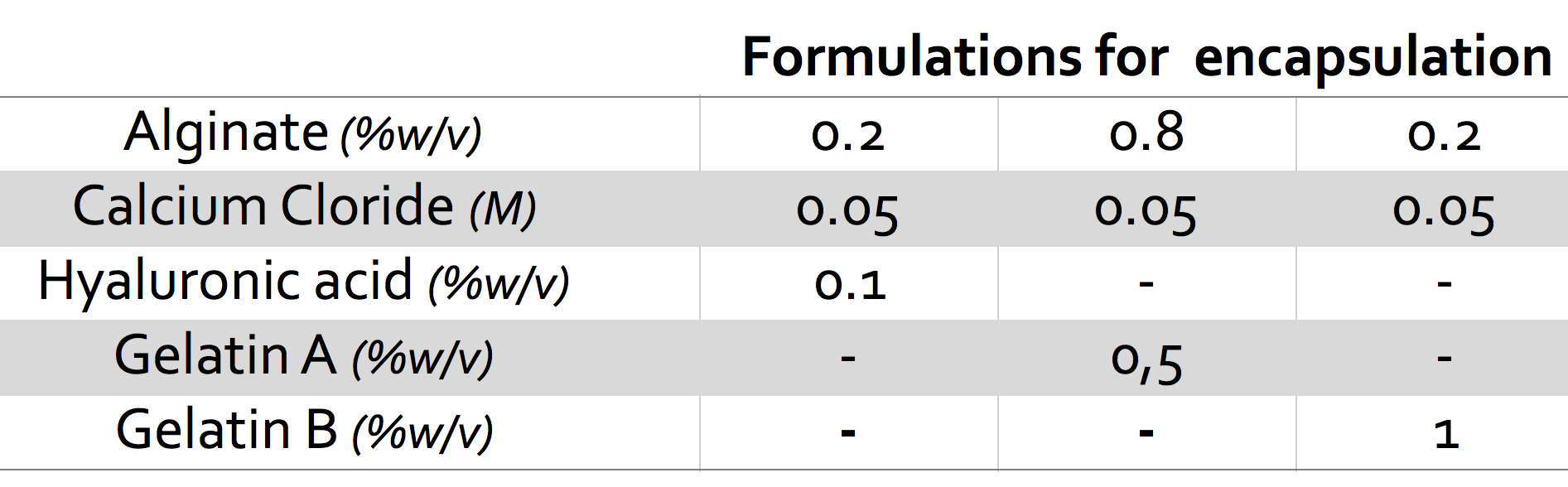
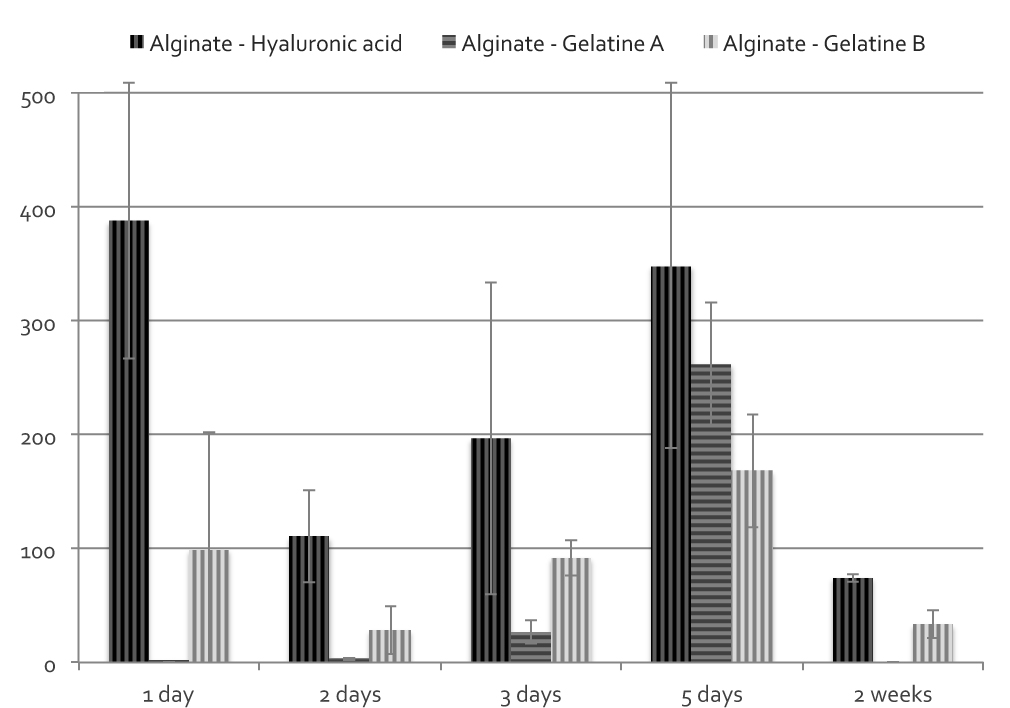
Despite similar weigh loss times, differences in cells release behavior were observed for the three compositions. Hyaluronic acid beads were found to release a significantly higher number of cells compared to gelatin formulations. Release of cells from gelatin A was concentrated in a very narrow period and more distributed for gelatin B and hyaluronic acid. For these latter, cell release was still observed after two weeks.
By combining alginate with selected macromolecules, is possible not only to adjust capsule degradation and control cell release, but it also possible to optimize beads properties and enhance cells survival and delivery efficiency to support soft tissue regeneration.
References:
[1] Reis RL, San Román J - Biodegradable Systems in Tissue Engineering and Regenerative Medicine – CRC Press, 2004
[2] Draghi L, Brunelli D, Farè S, Tanzi MC - Programmed cell delivery from biodegradable microcapsules for tissue repair - Biomater Sci Polym 26(15):1002-12