Introduction: Iron (Fe) and magnesium (Mg) are two type of biodegradable metals received much attention over the past 10 years[1]. Both were used for making coronary stents, while Mg was mostly proposed for bone implants[2],[3]. In view of enlarging the applicability of Fe for bone implants, we conduct this study to compare Fe with Mg as implant material in the in vivo setting using mice.
Materials and Methods: Adult male DDY mice were divided into Fe-group received pure Fe (∅=1 mm, length=10 mm) and Mg-group received pure Mg (∅=2.0 mm, length=10mm), both onto the femoral bone for up to 30 days. The implant degradation and its interaction with the local tissue and whole body system were assessed via peripheral blood profile, radiography and histology evaluation. Peripheral blood samples (0.5 mL) were taken from retro-orbitalis vein at day 0, 1, 10, and 30 post-implantation, then subjected to complete blood counting and to Fe and Mg ions level measurement using an atomic absorption spectrometer. Radiography imaging was taken at day 1, 7, 14, and 30 post-implantation using a Vet Digital Dental X-ray and evaluated for its radiodensity using ImageJ software. Histological analysis of the tissue surrounding implantation site was done at day 1 and 30 post-implantation.
Results and Discussion: Fig. 1a indicates the absence of hemolysis as red blood cell (RBC) number changed insignificantly over the period of implantation suggesting the hemocompatibility of Fe and Mg implants in mice[4]. The white blood cell (WBC) count (Fig. 1b) and neutrophyl/lymphocyte (N/L) ratio (Fig. 1c) as inflammatory and physiological stress biomarkers[5] indicate the occurrence of inflammation at day 1. At day 30, the Mg-group shows WBC decrease indicating an accumulation of the cells in the tissue instead of circulating in the system. The N/L ratio indicates physiological stress was more pronounce for Mg-group which can be related to larger volume of implant that stretched the muscle. Figs. 1c-d show a significant increase of Fe ion level indicating a pronounce effect of its degradation to the body capacity in balancing the additional Fe ions in the system. In Mg-group, the Mg ion level was well maintained in a narrow range and less than 1% of total Mg normally found in extracellular fluid[6].

Fig. 1: Results of peripheral blood profile evaluation: (a) RBC count, (b) WBC count, (c) N/L ratio, (d) Fe and Mg ions level. Note: Fe-Mg ion = Mg ion level in Fe-group.
Radiodensity analysis (Fig. 2) shows the occurrence of normal inflammatory reaction as marked by the increasing radiodensity surrounding the implantation site. The changes are in accordance with the stages of inflammation reaction and ends with connective tissue formation around the implant (higher radiodensity)[7].
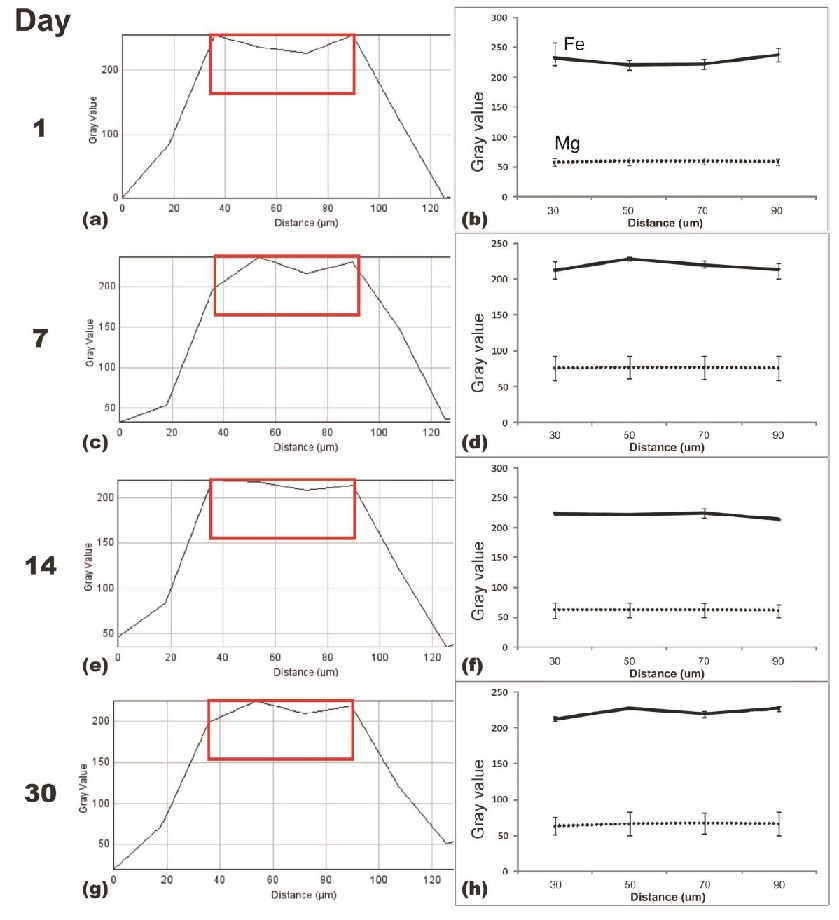
Fig. 2: Average radiodensity profile. Note: red boxes in a, c, e, g indicate the region of interest analyzed in b, d, f, h.
Histological analysis (Fig. 3) further confirms the acute inflammatory reaction by the appearance of inflammatory cells, edema and fibrin accumulation on the early days of implantation period. At day 30, a connective tissue with foreign body giant cells (fibrous capsule) was seen surrounding the Fe implant as well as its degradation product. Differently, this occurrence was not seen on tissue around Mg implant which corresponds with the higher WBC count at day 30 for Mg-group than Fe-group. This difference can be related to the fact that Fe degrades slower than Mg and moreover the formation of fibrous capsule limits the interaction between the Fe implant with the surrounding tissue[8].

Fig. 3: Histology of: (a, b, c) Fe-group, and (d, e, f) Mg-group. Note: Day 1 (a, d) and day 30 (b-f). Note: FB=femoral bone; BM=bone marrow; M=muscle; FC=fibrous capsule; star=implant site; arrow=foreign body giant cells.
Conclusion: This study shows that Fe and Mg degradation did not cause adverse effect in the in vivo setting in mice. Mg implant seems to induce more preferable tissue reaction than Fe implant. This study can be considered for the development of standardization of biodegradable metals testing.
The authors acknowledge Indonesian Ministry of Education and Culture-DGHE for grant No. 083/SP2H/PL/Dit. Litabmas/II/2015. The authors thank Dr. AK Nasution for preparing the implants. This work is part of the international Indonesia-Canada collaboration between Bogor Agricultural University and Laval University.
References:
[1] Y. F. Zheng, et-al., Materials Science and Engineering R 2014;77:1-34.
[2] H. Hermawan, D. Mantovani, Acta Biomaterialia 2010;6:1693-1697.
[3] J. Walker, et-al., Journal of Biomedical Materials Research B 2014;102:1316-1331.
[4] S-H. Ye, et-al., Langmuir 2013;29: 8320-8327.
[5] S. Balta, et-al., British Journal of Cancer 2013;109:3125-3126.
[6] M. Swaminathan, Clinical Biochemical Review 2003;24:47-66.
[7] D. Paramitha, et-al., Advanced Materials Research 2015;1112:466-469.
[8] T. Kraus, et-al., Acta Biomaterialia 2014;10:3346-3353.