Introduction: Understanding wear particle characteristics and their biological activity is an essential step for the pre-clinical testing of joint replacements. There is currently a lack of relevant studies on the biological response to ceramic wear debris due to difficulties in isolating and generating clinically relevant ceramic wear debris in vitro. Previous studies[1]-[3] tended to use commercially obtained ceramic particles to investigate the biocompatibility and reported limited or no reduction in cell viability upon exposure to commercial alumina ceramic particles. The wear performance of the latest composite zirconia-toughened platelet-reinforced alumina ceramic-on-ceramic bearings, otherwise commercially known as BIOLOX® delta has been extensively investigated[4],[5], however no studies have reported the characteristics or biological response to the wear debris generated from these bearings. The aim of this study was to determine the cytotoxicity of commercially obtained BIOLOX® delta powder compared with clinically relevant cobalt chromium (CoCr) nano-particles as an initial assessment of biocompatibility of model ceramic particles.
Materials and Methods: The commercially obtained BIOLOX® delta particles (CeramicTec, Germany) and clinically relevant CoCr wear particles (generated using a six station pin on plate wear simulator[6]) were initially characterised in terms of size, morphology and composition using a Hitachi SU8230 cold-field emission gun scanning electron microscopy (CFE-SEM), EDX analysis and image analysis software. The effect of CoCr and BIOLOX® delta particles on the viability of L929 fibroblasts was assessed using the ATP-Lite™ assay. The cells were cultured with 50 µm3 of CoCr nanoparticles or BIOLOX® delta ceramic particles at particle volumes ranging from 0.5 - 500 µm3 per cell and the cell viability was assessed over a period of 6 days.
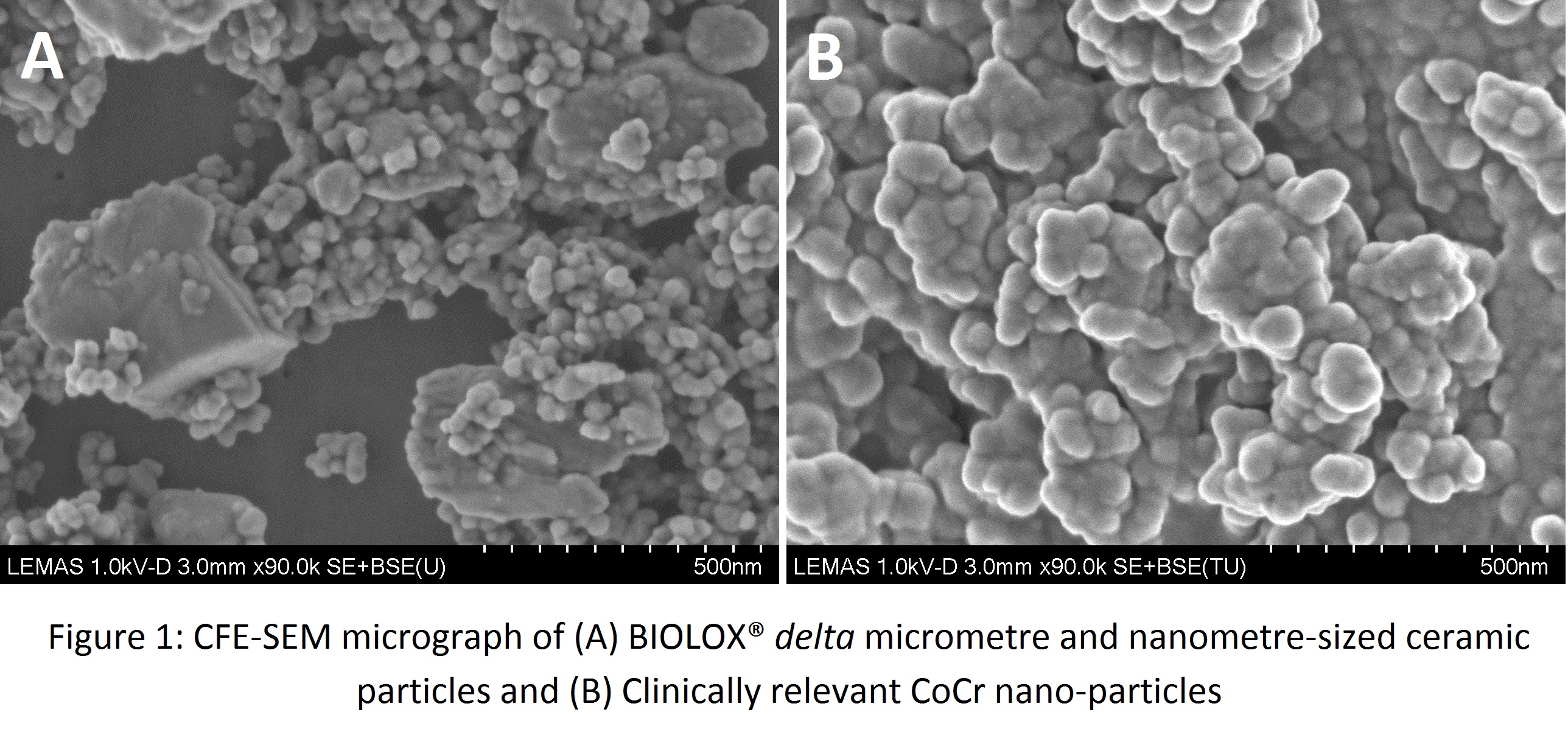
Results and Discussion: Characterisation of the particles showed that the BIOLOX® delta particles had a bimodal size range with large polygonal-shaped micron-sized alumina particles (0.5-2 µm) and small round nano-scale zirconia particles (60-70 nm; Fig 1A). The CoCr particles had a round morphology and were 50-130 nm in size (Fig 1B).
The BIOLOX® delta particles were mildly cytotoxic when compared with equivalent particle volumes (50 µm3) of clinically relevant CoCr particles. The CoCr particles (50µm3) had a significant cytotoxic effect from day 1, whereas the BIOLOX® delta particles significantly reduced the viability of the L929 fibroblasts (p<0.05 ANOVA) at particle concentrations of 50 and 500 µm3 after 6 days, when compared to the cell only negative control (Fig 2). The increased cytotoxicity of the CoCr particles compared to BIOLOX® delta particles may have been due to the release of Co and Cr ions.

Conclusion and Future Work: This study demonstrates the potential cytotoxic effects of model ceramic particles at very high volume concentrations, but it is unlikely that such high particle volumes will be experienced routinely in vivo in such low wearing bearing materials. Future work will include generation of clinically relevant BIOLOX® delta ceramic wear particles that will be used in subsequent biological assays to assess biocompatibility in terms of cytotoxicity, genotoxicity, inflammation and oxidative stress. This project will generate benchmark data on BIOLOX® delta that would be used for future material development.
Engineering and Physical Science Research Council (EPSRC); DePuy Synthes Joint Reconstruction
References:
[1] Catelas et al., Biomaterials, 1999. 20(7): 625-30
[2] Nkamgueu et al., J Biomed Mater Res. 2000.15; 52(4):587-94
[3] Tsaousi et al., Mutat Res, 2010. 29; 697(1-2):1-9
[4] Stewart et al., J Biomed Mater Res Part B Appl Biomater, 2003. 66B(2): p. 567-573
[5] Al-Hajjar et al., J Biomed Mater Res Part B Appl Biomater, 2013. 101(8): p.1456-62
[6] Papageorgiou et al., Nanomaterials, 2014. 4(2): 485-504