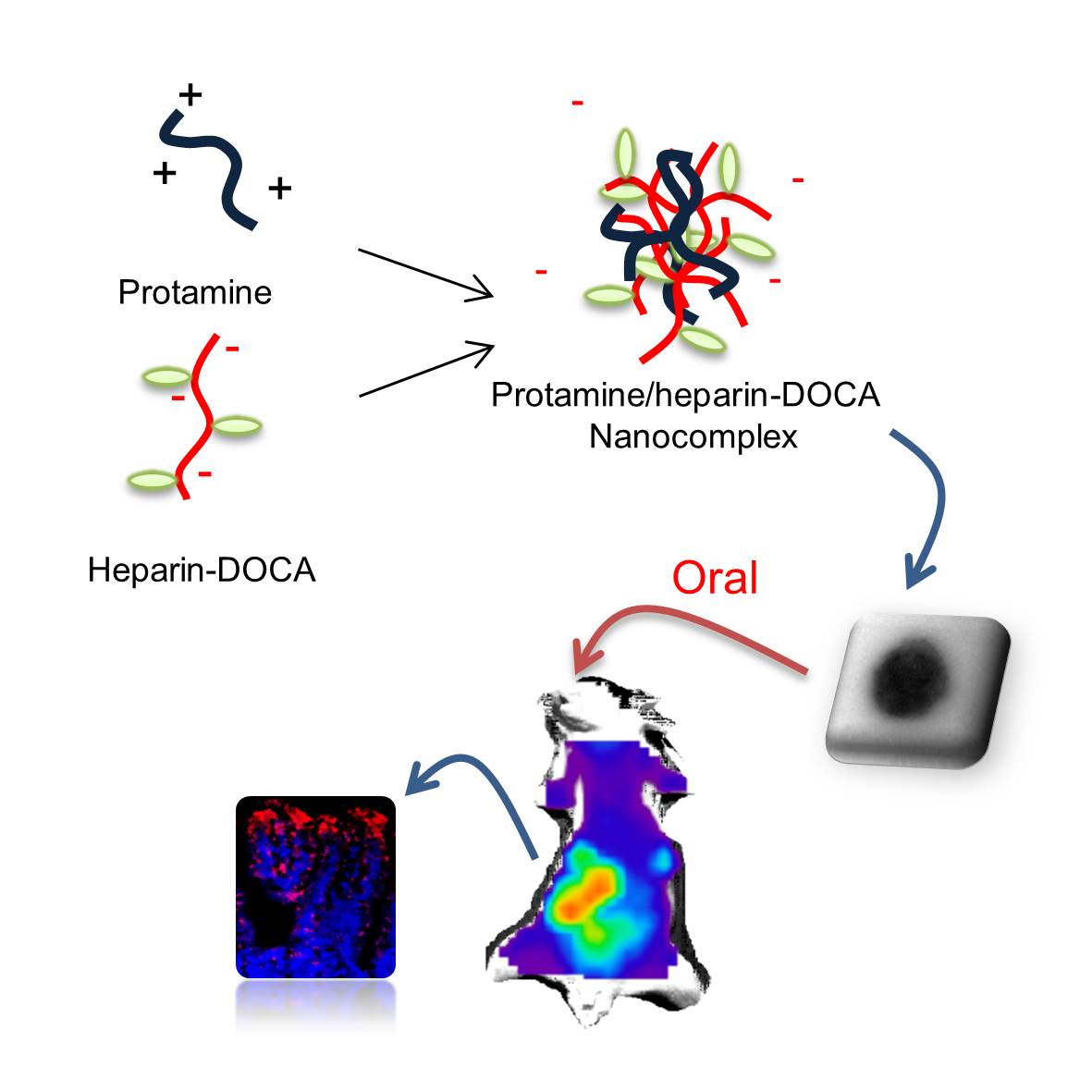
Oral delivery is the most clinically acceptable delivery route for patients. However, it is hard to deliver some therapeutic agents including macromolecule, peptide and gene. For decades, several researchers have tried to develop an oral agent for macromolecule delivery [1],[2]. Especially, nanoparticles and nanocomplex are remarkable carriers for therapeutic agents, but, they are not usually available as an oral tablet [3]. Here, we developed a new nanoparticle using two FDA-approved biomaterials for oral delivery. A negatively charged heparin and positively charged peptide named protamine have shown clinical therapeutic potential [4]. Using the polysaccharide, peptide and bile acids, we have designed an orally available nanoparticle. It was possible to control the surface charge and the size of nanoparticle by using the formulation of heparin and protamine. Moreover, the bile acids in nanoparticle enhanced the oral delivery of the nanoparticle. This nanoparticle could bind to the apical sodium-dependent bile acid transporter (ASBT) on caco-2 and MDCK cell. In addition, the nanoparticles were not in the tight junctions but they were present in the transcellular region; chitosan-based positive nanoparticles are usually absorbed via paracellular route. Furthermore, cy5-conjugated nanoparticle was treated to rats and mice to detect the nanoparticles in the GI tract. The orally treated nanoparticles were absorbed in the ileum, and then the retention period of the nanoparticles was also measured by using cy5 signals. In conclusion, these results represent an advanced major oral delivery technique for drug and gene delivery. The oral delivery of safe and functional nanoparticles would invoke the benefits of advanced oral delivery system providing a clinical therapeutic potential.
This study was supported by grants from the Bio & Medical Technology Development Program (grant no. 2012028833). The authors thank National Center for Inter-University Research Facilities for technical assistance.
References:
[1] Al-Hilal TA, Alam F, Byun Y. Oral drug delivery systems using chemical conjugates or physical complexes. Adv Drug Deliver Rev. 2013;65:845-64.
[2] Hwang SR, Byun Y. Advances in oral macromolecular drug delivery. Expert Opin Drug Deliv. 2014;11:1955-67.
[3] Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167.
[4] Thu MS, Bryant LH, Coppola T, Jordan EK, Budde MD, Lewis BK, et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat Med. 2012;18:463-7.