Nano-ordered and direct analysis of molecular interactions in protein adsorption process on polymer brush surfaces
-
1
the University of Tokyo, Department of Materials Engineering, Japan
Introduction: Protein adsorption is an initial and important event of biological responses, which progress hierarchically on a materials surface. Therefore, protein adsorption at interfaces should be precisely regulated for the control of bioreactions. The objective of this study is to analyze the protein adsorption process based on interaction forces generated at surfaces. Highly dense polymer brush surfaces with well-defined structures and properties were prepared using various monomers as platform surfaces to clarify the interaction forces operating at the surfaces. The interaction forces were evaluated by force-versus-distance (f-d) curve measurements with an atomic force microscope (AFM) using the probes modified with various molecules. The nano-ordered and direct analysis of molecular interaction forces at the polymer brush surfaces would lead to the accurate understanding for the protein adsorption process.
Experiment: Four kinds of the polymer brush surfaces with different physicochemical properties were prepared using 2-methacryloyloxyethyl phosphorylcholine (MPC, zwitterionic), 2-trimethylammoniumethyl methacrylate (TMAEMA, cationic), 3-sulfonate propyl methacrylate (SPMA, anionic), and n-butyl methacrylate (BMA, hydrophobic) (Fig. 1). The f-d curves between the same polymer brush surfaces were recorded using the AFM probes modified with the polymer brush layers. The interaction forces between the surfaces and proteins (albumin (pI 4.8) and lysozyme (pI 11.1)) in the phosphate-buffered saline at room temperature were evaluated using the proteins-immobilized AFM probe.
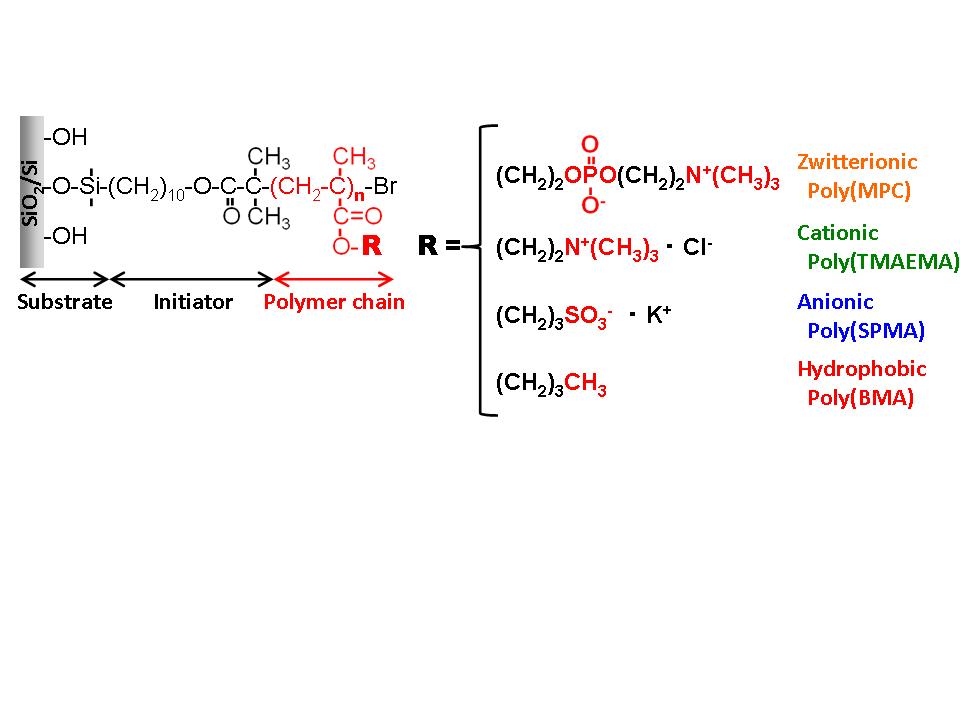
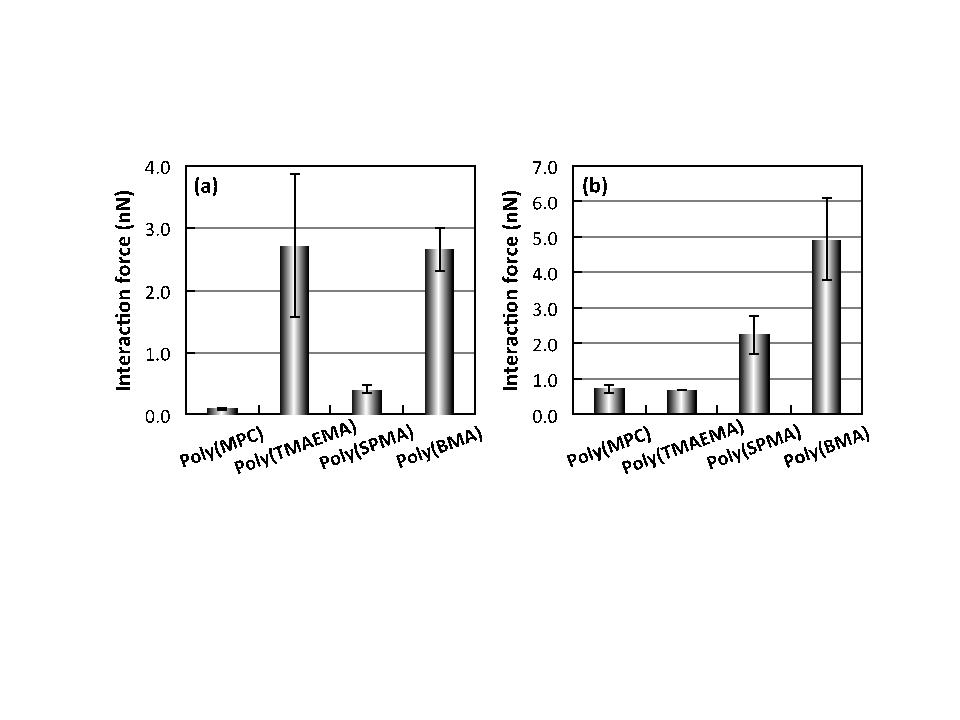
Results and Discussion: All the polymer brush surfaces had sufficient thickness and density enough to form a highly dense polymer brush structure (> 0.10 polymer chains/nm2). The properties of the polymer brush surfaces corresponded to those of the monomer units. The poly(TMAEMA) and poly(SPMA) brush surfaces showed large repulsion forces in water during the approaching process, and the repulsion forces were weakened with the increase in the ionic strength, indicating the electrostatic interactions. The poly(BMA) brush surface induced a strong attractive force during the retracting process, which is based on the hydrophobic interaction. On the other hand, the poly(MPC) brush surface had no specific interaction during both processes. As shown in Fig. 2, the interaction forces between poly(MPC) brush surface and proteins were quite low (< 1.0 nN), while the poly(TMAEMA) and poly(SPMA) brush surfaces strongly interacted with proteins with opposite net charge, and the poly(BMA) brush surface strongly interacted with both proteins. Furthermore, the interaction forces were not generated during the approaching process of protein toward the surface. From these results, we concluded that the electrostatic and hydrophobic interactions would not work as the driving force to attract proteins to the surface, but prevent the reversible detachment of proteins from the surfaces.
Keywords:
Biocompatibility,
Surface modification,
polymer brush,
bioinerface
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Protein interactions with biomaterials
Citation:
Inoue
Y,
Sakata
S and
Ishihara
K
(2016). Nano-ordered and direct analysis of molecular interactions in protein adsorption process on polymer brush surfaces.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00828
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.