Introduction: Hydroxyapatite (HAP, Ca10(PO4)6(OH)2) is a well-known bioceramic with superior biocompatibility, excellent affinity to host hard tissues and good osteoconductivity[1]. Synthetic HAP which has similar properties to the mineral phase of natural bone, has been widely studied for use in bone tissue engineering and drug delivery [2]. Incorporation of HAP nanoparticles into a polymeric matrix has been a successful method to enhance bioactivity and mechanical properties of the composite [3]. For applications in composite materials for drug delivery, mono-disperse HAP nanoparticles with good stability in the polymer solution are required [4]. Even though numerous works have been done in controlling morphology of the particles, [5] it is still a challenge to prepare nano-sized crystals with good colloidal stability. Moreover, poorly crystalline HAP crystals are particularly beneficial in terms of their high specific surface area, biodegradability, binding ability to various molecules and improved cell proliferation [6].In this study, HAP nanoparticles were synthesized by modifying wet chemical methods from previous reports [7],[8].
Materials and Methods: In the first method precipitation with quick mixing was used as reported by Zhang and Lu [7]. This method applies addition of 0.5 M Ca(NO3)2.4H2O ethanol solution to 0.5 M (NH4)2HPO4 water solution, all at once, with vigorous stirring at 40 °C at pH 11. In the present work, reaction time and aging were modified. The approach by Swain and Sarkar [8] was modified and involved slow addition of aqueous solutions of the same salts followed by NH4OH addition to reach pH 10. XRD and FTIR analysis were used to identify phases and carbonate substitution. TEM was used to analyse the morphology and BET to measure the surface area.
Results and Discussion: XRD and FTIR analysis confirmed that the HAP particles produced had a low % carbonate inclusion as well as a low crystallinity. In general, temperature, reaction or aging time, and pH of the solution are key parameters in controlling the morphology of HAP nanoparticles [5]. The use of ethanol solvent for the calcium reagent was aimed at having supersaturation of the solution with respect to HAP [7]. In addition, in this work, it was found that the mixing method, whether slow or rapid, play an important role in determining the morphology of HAP. TEM images in the Figure show that HAP particles from a quick mixing method (Image A) are single non-agglomarated particles; 5 - 20 nm in width and 15 - 40 nm in length. For the slow mixing method (Image B) it can be clearly seen that the particles are highly agglomerated with low aspect ratio.
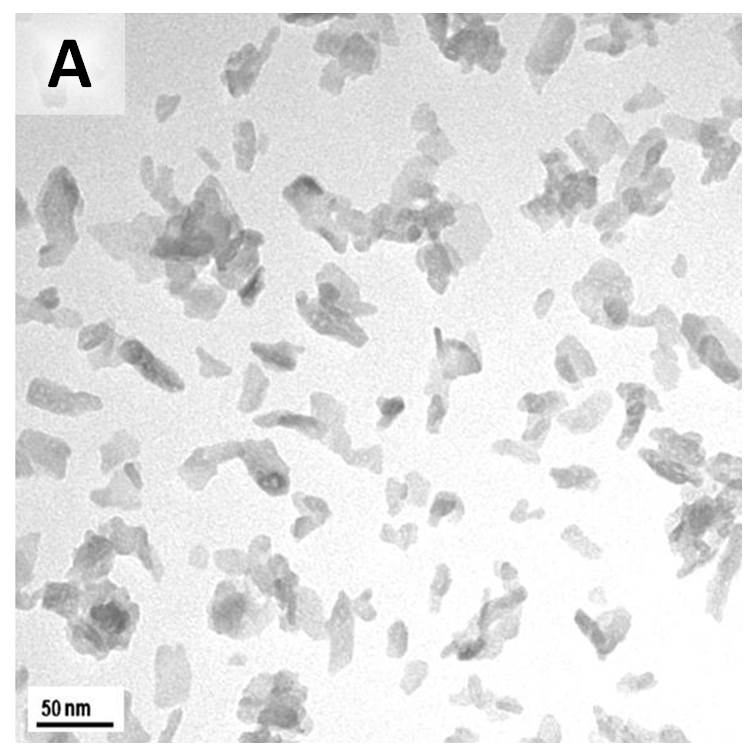
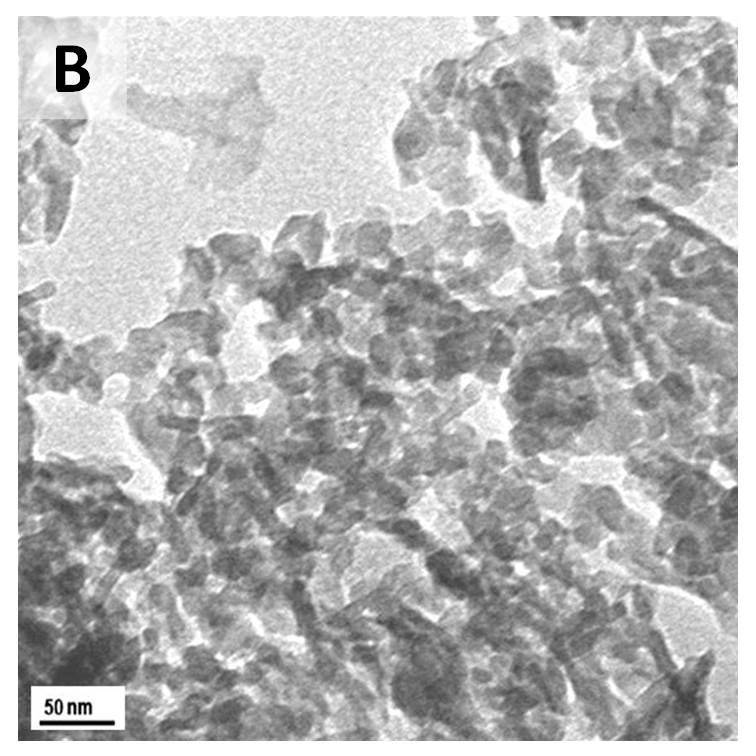
The highly dispersed nanoparticles obtained from the quick mixing method are more suitable for fabrication of composite materials. It has been suggested that quick mixing facilitates a uniform nucleation of HAP nanoparticles and limits the preferential growth of HAP crystal [7].
Conclusion: Quick mixing appears to be a key factor in obtaining HAP nanoparticles with low crystallinity and with low aspect ratio which are well-dispersed suitable for drug delivery application.
This work was carried out in part in the Centre for Microscopy and Microanalysis, The University of Queensland, the Queensland node of the Australian Microscopy and Microanalysis Research Facility (AMMRF).; This research is a part of doctoral degree of the first author therefore we acknowledge the Ministry of Research and Technology Republic of Indonesia for a scholarship.
References:
[1] H. Zhou and J. Lee, Nanoscale hydroxyapatite particles for bone tissue engineering, Acta Biomaterialia 7 (2011) 2769–2781
[2] S. Bose and S. Tarafder, Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review, Acta Biomaterialia 8 (2012) 1401–1421
[3] K.S. Jack, S.Velayudhan, P. Luckman, M. Trau, L. Grøndahl, and J. Cooper-White, The fabrication and characterization of biodegradable HA/PHBV nanoparticle–polymer composite scaffolds, Acta Biomaterialia 5 (2009) 2657–2667
[4] X. Jin, J. Zhuang, Z. Zhang, H. Guo, and J. Tan, Hydrothermal synthesis of hydroxyapatite nanorods in the presence of sodium citrate and its aqueous colloidal stability evaluation in neutral pH, Journal of Colloid and Interface Science 443 (2015) 125–130
[5] M. Sadat-Shojai, M.T. Khorasani, E. Dinpanah-Khoshdargi, A. Jamshidi, Synthesis methods for nanosized hydroxyapatite with diverse structures, Acta Biomaterialia 9 (2013) 7591–7621
[6] R. Bosco, M. Iafisco, A. Tampieri, J.A. Jansena, S.C.G. Leeuwenburgha, J.J.J.P. van den Beuckena, Hydroxyapatite nanocrystals functionalized with alendronate as bioactive components for bone implant coatings to decrease osteoclastic activity, Applied Surface Science 328 (2015) 516–524
[7] Y. Zhang and J. Lu, A simple method to tailor spherical nanocrystal hydroxyapatiteat low temperature, Journal of Nanoparticle Research, (2007) 9:589–594
[8] S.K. Swain and D. Sarkar, A comparative study: Hydroxyapatite spherical nanopowders and elongated nanorods, Ceramics International 37 (2011) 2927–2930