Introduction: Cross-linked, anti-oxidant stabilized UHMWPE is preferred in total hip replacements due to its wear and oxidation resistance [1]. Cross-linking can be achieved by radiation or using chemical cross-linkers like peroxides. High temperature melting (HTM) of radiation cross-linked UHMWPEs around 300°C was shown to improve toughness by inducing controlled chain scission and enhanced intergranular diffusion of chains simultaneously [2]. In this study we combined vitamin E blending, peroxide cross-linking and high temperature melting to obtain a highly wear and oxidation resistant cross-linked UHMWPE with superior toughness. We also optimized the large-scale manufacturing process and tested the biocompatibility.
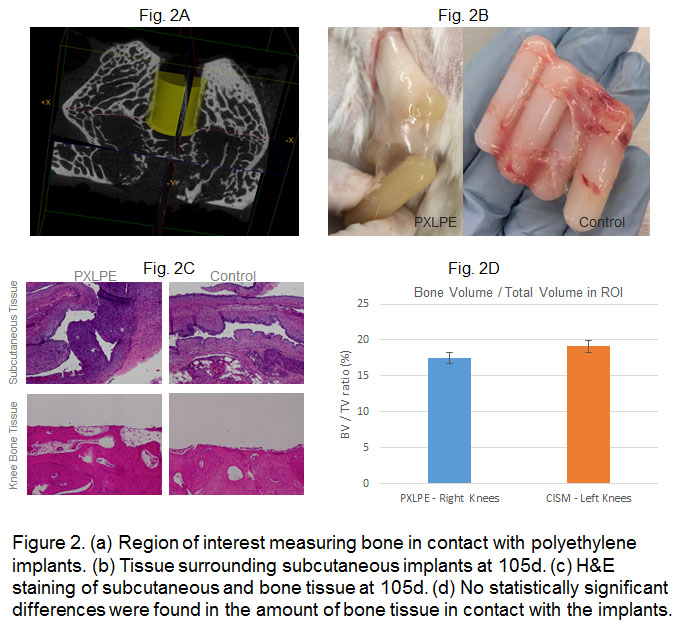
Materials and Methods: Medical grade GUR1050 UHMWPE was blended with vitamin E (0.2 wt%) and with 2,5-Di(tert-butylperoxy)-2,5-dimethyl-3-hexyne or ‘P130’ (0.8 wt%) and consolidated by ram extrusion into 4” diameter and 5ft. long cylinders. These blocks (4” dia, 8cm thick) underwent an optimum high temperature melting (HTM) cycle (PRX-HTM; Figure 1a). The blocks were tested for wear rate, impact strength, tensile properties (UTS and EAB) and oxidation induction time (OIT) 'as is' or after 25 kGy gamma sterilization. In vivo biocompatibility tests were done by implanting the PRX-HTM (1cm dia x 3.75 cm length implant) subcutaneously in the dorsum of rabbits for 105 days. Tissue in subcutaneous pockets was evaluated histologically.
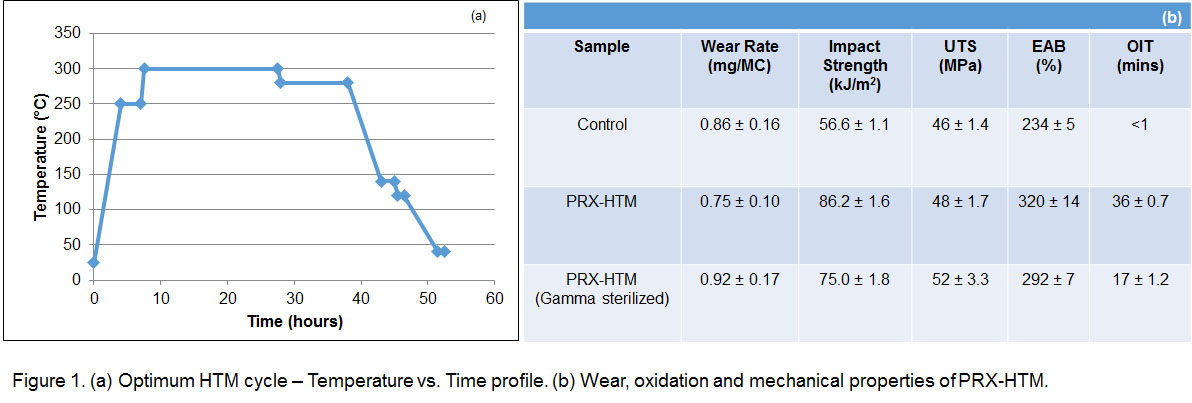
Results: The strength, toughness and oxidation resistance of the PRX-HTM was superior compared to the clinically relevant control (Virgin UHMWPE irradiated to 100 kGy and subsequently melted), without sacrificing the wear rate. The material showed no significant changes after gamma sterilization (Figure 1b). No adverse reactions were encountered in vivo subcutaneously after a 105 day implantation of the PRX-HTM (Figure 2).
Discussion: We were able to successfully scale up the PRX-HTM material from laboratory to large scale manufacturing without sacrificing any properties and demonstrating local implant biocompatibility. This was achieved by processing the peroxide cross-linked UHMWPE to an optimum HTM cycle (Figure 1a) specially designed to improve the properties and reduce the residuals formed during peroxide decomposition. This material showed promise by providing the ability to consolidate and cross-link the UHMWPE in a single step which would lead to substantial cost reduction by eliminating high dose irradiation. This is a breakthrough implant material showing tremendous improvement over currently available alternatives.
Conclusions: Large scale peroxide cross-linking of UHMWPE followed by high temperature melting yields a feasible implant material with improved toughness without sacrificing the wear resistance.
References:
[1] Oral et al. Biomaterials (2008) 29:3557-60
[2] Fu et al. Polymer (2010) 51:2721-31