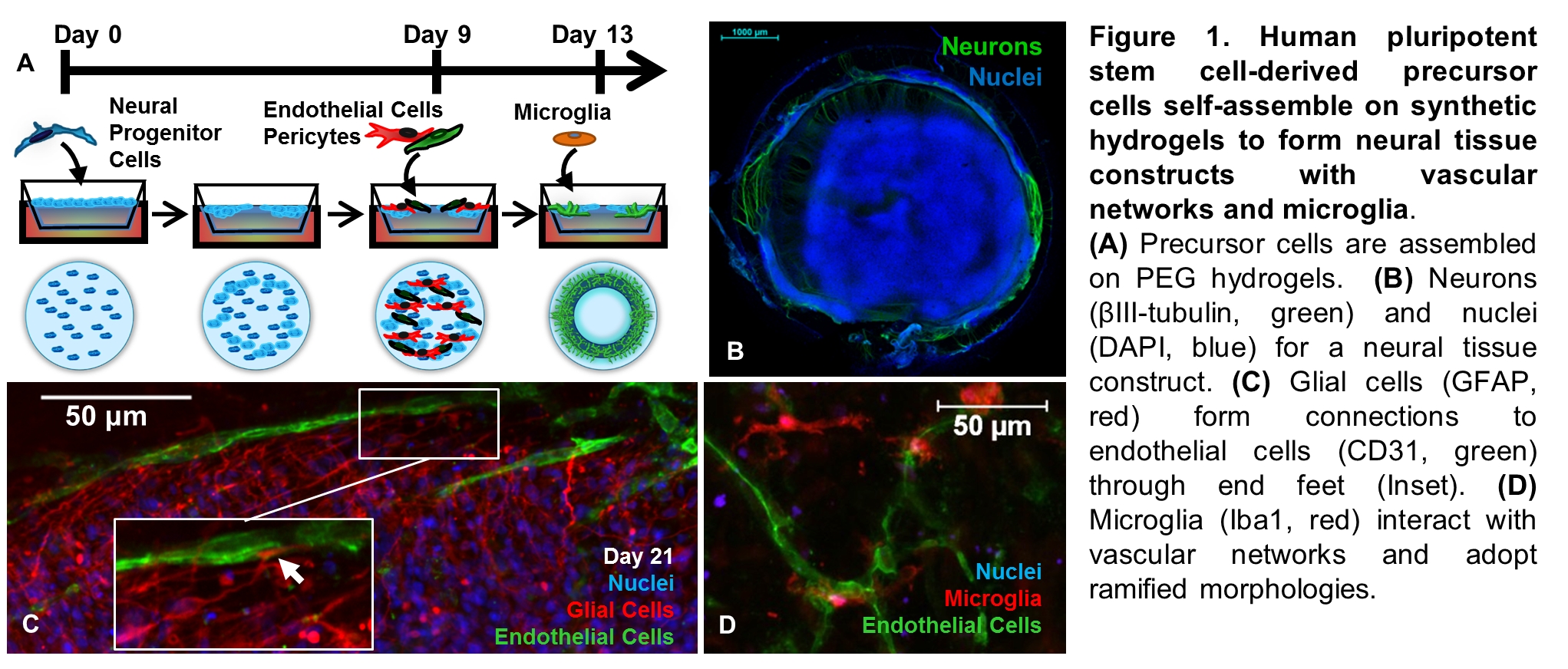
Animal testing is costly, controversial, and provides limited predictive value for human neurotoxicity[1]. In vitro cellular models that reflect human physiology offer an alternative to animal testing for assessing the efficacy and potency of pharmaceuticals, and for predicting potential toxicities for drugs, industrial chemicals, and food additives[1]. To address these needs, two neural tissue models were produced by culturing human pluripotent stem (PS) cell-derived neural progenitor cells (NPCs) on poly(ethylene glycol) (PEG) hydrogels formed using “thiol-ene” photopolymerization (Fig. 1a)[2], as previously described in detail[3],[4].
For the first model, vascularized neural tissue constructs with microglia are formed by first culturing human PS cell-derived NPCs on PEG hydrogels in 24-well Transwell inserts (Fig. 1A). The PEG hydrogels are designed with protease-degradable peptide crosslinks and incorporate cell adhesion peptide to enable cellular remodeling[2]. NPCs self-assemble into multilayered tissues with radially organized neuronal and glial cells reminiscent of the early neuroepithelium when cultured on these peptide-functionalized PEG hydrogels[3]. Human PS cell-derived vascular and microglial precursor cells are added after initial self-organization of NPCs (Fig. 1A) to mimic in vivo recruitment of blood vessels and microglia after formation of the neural tube[3].
The procedure produces neural tissue constructs with diverse neuronal phenotypes (Fig. 1B), interconnected vascular networks (Fig. 1C), and microglia with characteristic ramified morphologies (Fig. 1D). We previously demonstrated that the neural tissue constructs were reproducible by RNA-sequencing and could be used to predict neurotoxicity with 90% accuracy in a blinded trial[3].

For the second procedure, human induced pluripotent stem cell (iPSC)-derived NPCs (iPSC-NPCs) are differentiated and matured on PEG hydrogels (Fig. 2A) to optimize neuronal phenotypes for botulinum neurotoxin (BoNT) detection[5]. Despite being the most potent known human toxin, BoNT/A1 is widely used for cosmetic and pharmaceutical applications[5]. Each new BoNT/A1 batch must therefore be assessed for potency, typically using the in vivo "mouse bioassay" to determine an LD50 dose (the dose that causes 50% lethality after 4 days)[5]. Human iPSC-NPCs that are differentiated and matured on PEG hydrogels are characterized by higher gene expression for mature neuronal phenotypes than cells cultured on tissue culture polystyrene (TCP)[4]. Importantly, iPSC-NPCs that are differentiated and matured on PEG hydrogels detect BoNT/A1 at sensitivities comparable to the mouse bioassay earlier than cells cultured on TCP (Fig. 2B)[4].
Taken together, our results demonstrate that PEG hydrogels provide an alternative to naturally-derived materials such as Matrigel for inducing self-assembly of human PS cell-derived neural progenitor cells into model 3D neural tissues that are well-suited for neurotoxicity screening (including toxicity and potency). The neural tissue models described here are formed using standard cell culture techniques and labware that can be adapted for robotic liquid handling, and efforts are underway to automate these procedures.
National Institutes of Health (1UH2TR000506-01, 3UH2TR000506-02S1, 4UH3TR000506-03, R21EB016381-01, R01AI095274, R01AI093504)
References:
[1] Bal-Price A, Crofton K, Leist M, Allen S, Arand M, et al. (2015) International stakeholder network (istnet): Creating a developmental neurotoxicity (dnt) testing road map for regulatory purposes. Arch Toxicol 89: 269-287.
[2] Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, et al. (2009) A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater 21: 5005-5010.
[3] Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, et al. (2015) Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci USA doi: 10.1073/pnas.1516645112.
[4] Pellett S, Schwartz MP, Tepp WH, Josephson R, Scherf JM, et al. (2015) Human induced pluripotent stem cell derived neuronal cells cultured on chemically-defined hydrogels for sensitive in vitro detection of botulinum neurotoxin. Sci Rep 5: 14566.
[5] Pellett S (2013) Progress in cell based assays for botulinum neurotoxin detection. In: Rummel A, Binz T, editors. Botulinum neurotoxins. Berlin: Springer-Verlag Berlin. pp. 257-285.