Introduction: Breast cancer is the most common cause of cancer related deaths in women worldwide and metastasis accounts the vast majority of such deaths[1]. Metastasis is a multi-step process wherein the cancer cells migrate from the primary tumor into the vasculature, survive in the circulation, extravasate into the secondary site and establish a tumor[2]. Understanding the molecular mechanisms that orchestrate metastasis provide useful insights into developing therapeutics that prevent or treat metastasis. While conventional two-dimensional (2D) cell culture systems fail to mimic in vivo signaling due to the planar architecture and inappropriate substrate stiffness[3], animal based xenograft models are expensive and time consuming. To overcome these drawbacks, three dimensional (3D) scaffold based models are being developed which help recapitulate the in vivo tissue micro-environment. In this study, for the first time, we have developed a comprehensive in vitro model to study the events in breast cancer metastasis using a 3D scaffold of polycaprolcatone (PCL).
Materials and Methods: Macro-porous scaffolds of PCL were prepared by salt leaching with a pore size of 250-400 µm. MDA-MB-231, a transformed metastatic breast cancer cell line was cultured on the PCL scaffolds and compared to cells cultured on conventional 2D TCPS dishes. Gene expression was scored using quantitative Real Time PCR. Protein expression was assayed using immuno-fluorescence. Functional assays like invasion and sphere formation in methyl cellulose were performed to assess the invasion potential and cancer stem cell activity. In vivo tumorigenicity and metastatic potential were assayed. Global gene expression changes were analyzed using whole genome gene expression microarrays.
Results and Discussion: 3D porous PCL scaffolds were fabricated to mimic the mechanical properties of the breast tissue. The topography of the scaffold mimicked the acinar architecture and the elastic modulus was 7.5 kPa, comparable to that of the breast tumor[4]. MDA-MB-231 cells cultured on the scaffolds formed a 3D cell-cell contacts which on prolonged culture gave rise to tumoroids. Scaffold cultured cells had higher expression of genes like TWIST1, Vimentin, Nanog, Oct3/4, CD44, Fibronectin, Gli2 and PTHrP which are implicated in initiation, progression and colonization of the metastasis cascade. Functional assays showed increased invasion and sphere formation in scaffold cultured cells compared to cells grown in control dishes. The scaffold cultured cells exhibited increased tumor formation ability and increased lung metastasis in NOD/SCID mice, suggesting that culturing cells on PCL scaffolds increased their metastatic potential compared to culturing on conventional TCPS dishes. Microarray based whole genome transcript analysis also supported the qRT PCR data and indicated a profound increase in cell-cell and cell-matrix interactions in the scaffold cultured cells. Also the analysis revealed that these cells had a strong pro-inflammatory gene expression signature which could play an important role in tumor-stroma interactions and tumor metastasis.
Conclusion: 3D PCL scaffolds can be used for culture of breast cancer cells to mimic many of the molecular events of breast cancer metastasis in contrast to cells on conventional culture plates. The cells in scaffolds exhibit enhanced metastatic potential in vitro which resulted in more potent tumor in vivo.
Acknowledgement: We thank the Council for Scientific and Industrial Research (CSIR), India, for funding KC for this work.
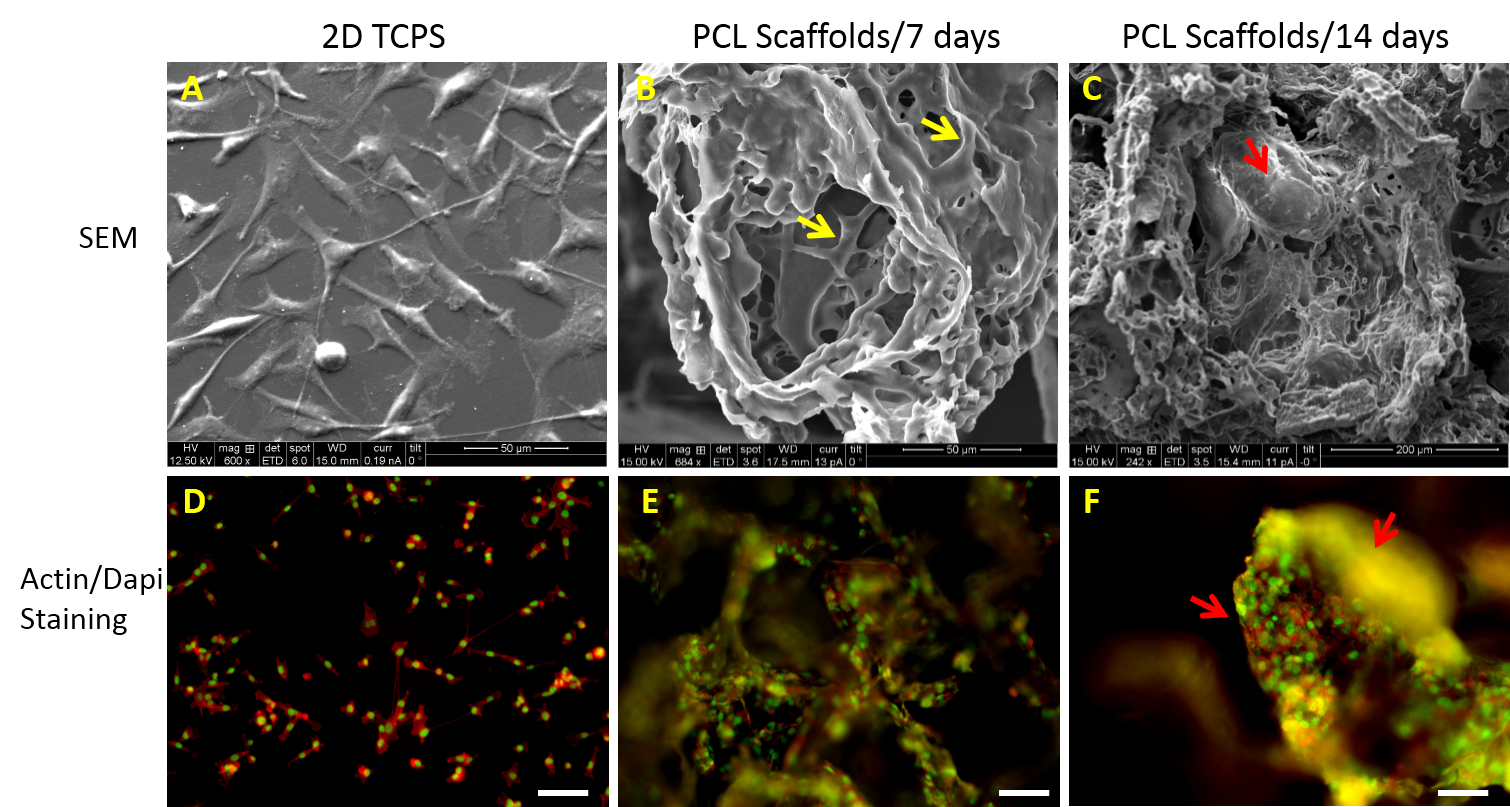
Figure 1: SEM micrographs of MDA-MB-231 cells in 2D TCPS (A) and in 3D scaffolds cultured for 7 (B) and 14 days (C). Yellow arrows indicate inter-cellular contacts. Tumoroid mass formed by MDA-MB-231 cells on culture for 14 days indicated by red arrows. Fluorescence images of MDA-MB-231 cells in 2D TCPS (D) and in 3D scaffolds cultured for 7 (E) and 14 days (F) stained for actin (red) and nucleus (green). Scale bars represent 50 microns.

Figure 2: Live animal imaging of tumors formed by MDA-MB-231 cells from control TCPS dishes and from 3D PCL scaffolds (SDT) in NOD-SCID mice.
References:
[1] Bendre M, Gaddy D, Nicholas RW, Suva LJ. Breast Cancer Metastasis to Bone: It is Not All About PTHrP. Clin Orthop Relat Res 2003;415.
[2] Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274–84. doi:10.1038/nrc2622.
[3] Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A 1998;95:14821–6.
[4] Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005;8:241–54. doi:10.1016/j.ccr.2005.08.010.