Introduction: Since directed cell movement is vital for controlling the tissue regeneration process, understanding the relative influences of structural factors on cell migration is crucial for bioactive scaffold design. Using structural parameterization by Micro-CT, we have recently shown that a critical interconnectivity threshold must be overcome for efficient fibroblast invasion into collagen scaffolds[1]. Here, we investigate the mechanism behind this effect by examining cell morphology and migration in response to controlled interconnectivity variation. We therefore demonstrate the importance of interconnectivity for the design and development of scaffolds for optimized cell migration.
Methods: Collagen scaffolds were fabricated by freeze-drying acidic suspensions of type I bovine collagen at 1 wt%. Interconnectivity variation was achieved by control of suspension composition and freezing conditions, and characterized along with pore size using X-ray Micro-Computed Tomography (Micro-CT), as previously described[1]. Interconnectivity was parameterized by calculating the size of the largest sphere able to travel through an infinitely large scaffold: the percolation diameter, dc[1]. Cell response was investigated by seeding scaffolds with human primary fibroblasts, at 15,000 cells in 1 µL medium (unlabelled) or 20,000 cells in 20 µL medium (fluorescently labelled) per scaffold. Scaffolds with labelled cells were transferred to a Yokogawa CV1000 Cell Voyager microscope system at day 1 after seeding. Cell migration was observed in real-time using time-lapse imaging, with images taken every 2 hours over a 42 hour period. Unlabelled cells were cultured to day 3, before staining with Phalloidin and DAPI. Cell position and morphology were assessed from fluorescent images taken in cross-section.
Results and Discussion: Calculation of mean cell speed revealed a strong correlation with scaffold interconnectivity, as shown in figure 1. However, increasing the interconnectivity above approximately dc = 60 µm produced no further increase in cell speed, which reached a plateau of 25 µm/hour. Even above this threshold, however, enhanced interconnectivity had a measurable influence on cell behaviour, as shown in figure 2. Scaffolds with higher interconnectivity permitted a greater extent of cell elongation, resulting in more persistent motion, and therefore improved coverage of distance over the 42 hour imaging period.
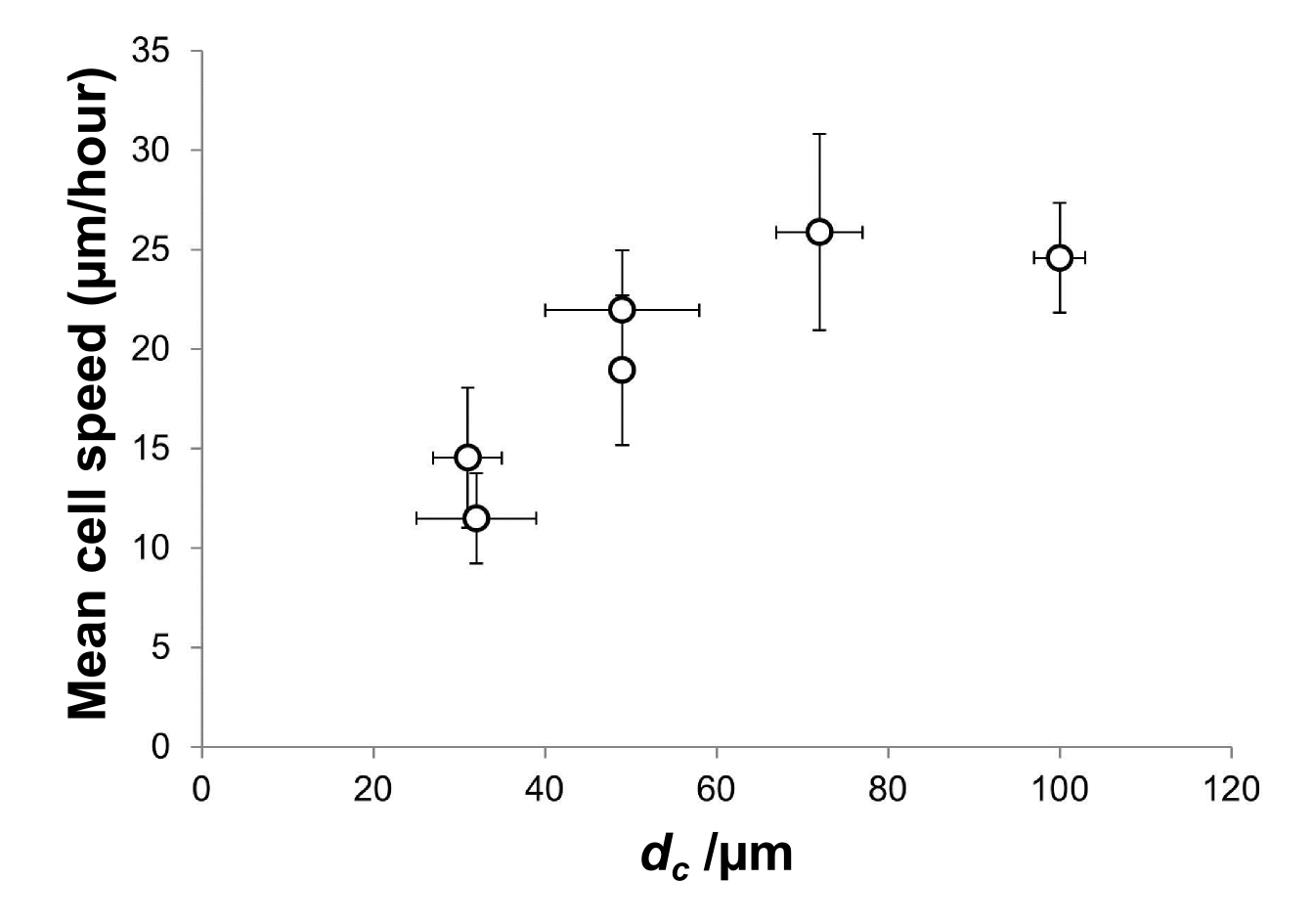
Figure 1: Mean speed of 10 cells as a function of interconnectivity, dc, shown with standard error.
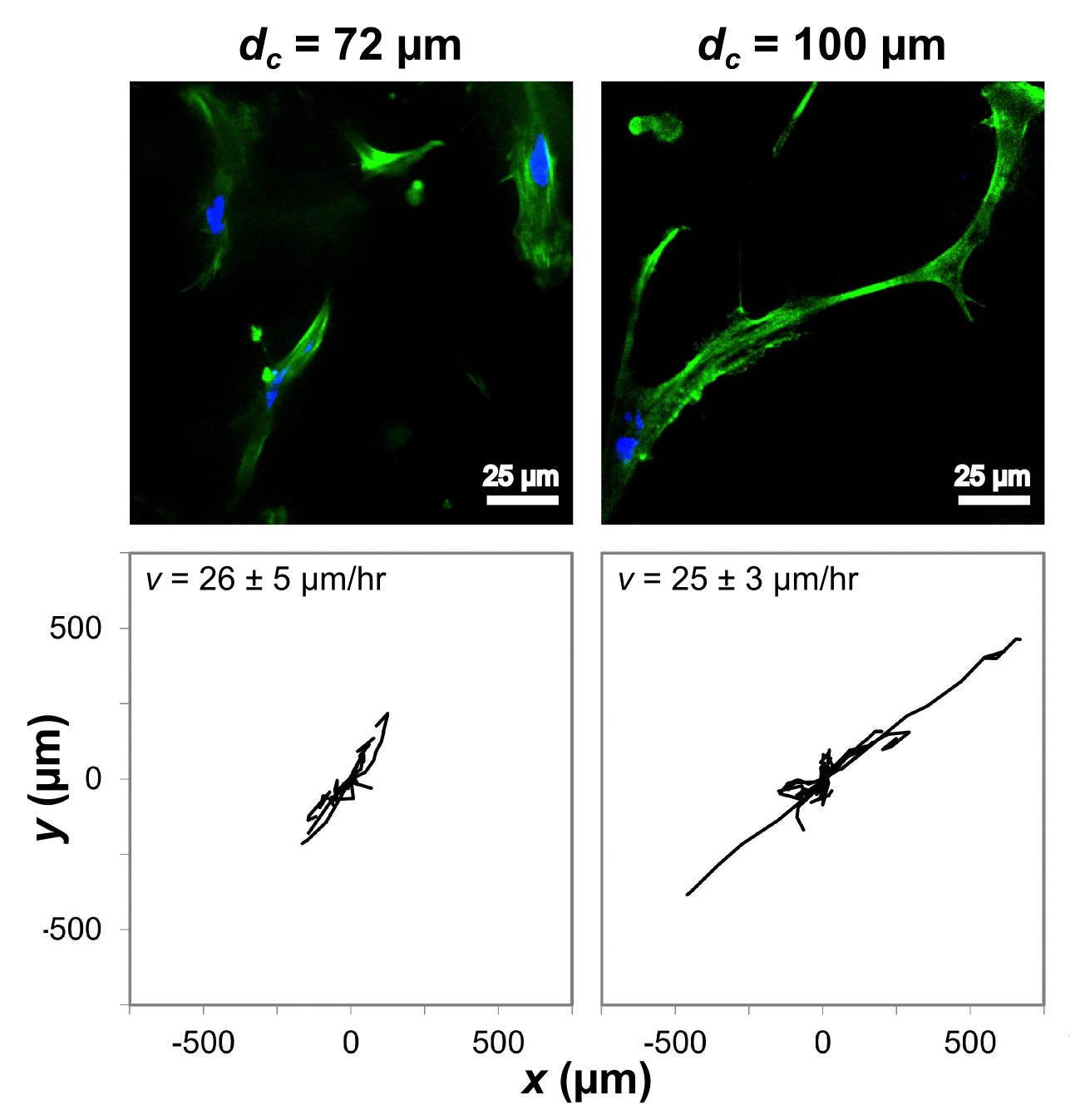
Figure 2: Cell morphology (top) and motion tracks of 10 cells (bottom) as a function of interconnectivity, dc, in scaffolds of constant pore size and anisotropy.
Cell speed at pore wall junctions is known to be lower than the speed along the pore walls[2]. It is therefore likely that a high interconnectivity structure decreases the time spent at junctions, as well as allowing more efficient spreading and elongation along the collagen pore walls: a factor previously reported to be important for directional cell motion[3]. Interconnectivity optimization therefore enhances both cell speed and directionality, and is a vital concept in the design of scaffolds for directed cell motion.
Conclusions: Using a combination of live-cell imaging and Micro-CT, we have demonstrated the importance of interconnectivity for enhancing cell migration, in terms of both speed and motion persistence.
The authors acknowledge financial support from the Engineering and Physical Sciences Research Council (EPSRC), Geistlich Pharma AG and European Research Council (ERC) Advanced Grant 320598 3D-E.
References:
[1] Ashworth, J. et al., Advanced Healthcare Materials, 4:1317-21, 2015
[2] Harley, B. et al., Biophysical Journal, 95:4013-24, 2008
[3] Caliari, S. & Harley, B., Biomaterials, 32:5330-40, 2011