Introduction: Biodegradable magnesium-based materials have a high potential for cardiovascular stent applications because most current conventional stents are made of non-degradable metals and polymers, which may negatively interact with the surrounding tissues to induce the chronicpathological response such as late thrombosis, delayed endothelialization and local chronic inflammation[1],[2]. However, there still exist concerns on how to control magnesium-based materials corrosion behavior to improve their biocompatibility[3]. In this paper, Mg alloys and surface modified Mg alloys are manufactured and their degradation behaviors in microfluidic system as well as in blood vessel are investigated. Their potentials as cardiovascular stent are also evaluated.
Methods and Materials: Preparation of material and coating: High-purity Mg alloy AZ31(MgZnMn) with 1 wt.% zinc (Zn) and 0.2 wt.% manganese (Mn) was prepared by melting and extruding to rod shape. The Magnesium wires with 10mm in length, 270 um in diameter were acquired by drawing process for degradation test in vivo and vitro. PTMC (–(COOCH2CH2CH2–O)n–) with an average molecular weight of 500,000 g mol-1 were dissolved in dichloromethane at a concentration 0.5wt%[4]. The polymer coatings on the alloy were fabricated via evaporation of the organic solvent from the coating solution. Uncoated Mg alloys specimens were treated as controls.
Dynamic degradation test: Dynamic degradation tests were conducted on a microfluidic system designed to mimic the environment encountered by stents in coronary arteries with a constant laminar flow[5]. During the test intervals, the corresponding pH value of the solution and the concentration of Mg2+ in the solution were respectively measured by pH meter and inductively coupled plasma atomic emission spectrometry. The morphology of the specimens was observed by SEM.
In vivo biocompatibility: To assess biocompatibility and real degradation behavior of an implant in coronary arteries, uncoated, PTMC-coated Mg alloys wires were implanted in rat’s coronary arteries. Samples were acquired after 15 days implantation. Samples were stained with hematoxylin and eosin and histological evaluation was developed.
Result and discussion: The dynamic degradation rate of the PTMC-coated Mg alloys was significantly lower than that of uncoated Mg alloy which also exhibit the property to increase of pH value and weight loss during the degradation Fig.1).
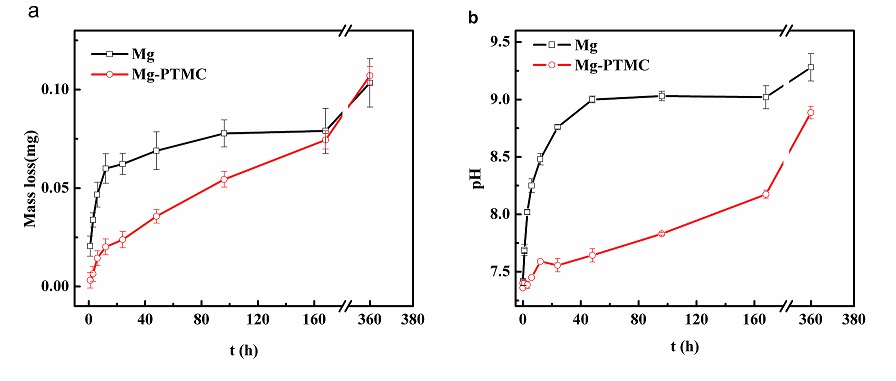
Fig1.The dynamic degradation of the uncoated and PTMC-coated samples in SBF at different stage: (a) weight loss of the specimens;(B) pH value of SBF
Corrosion morphologies for each type of samples at different dynamic degradation stage presented (Fig. 2). The surface morphologies of all samples were fairly smooth and clean before and after coating. After 168h of the degradation, some corrosion products and cracks emerged on the uncoated Mg alloys surface. For the PTMC coated samples, The PTMC swelling phenomenon was observed indicating the PTMC film can resist the corrosion of Mg alloys in some degree.
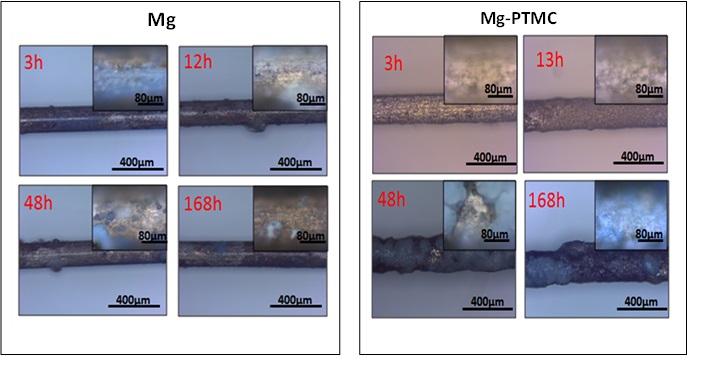
Fig2. Representative SEM images of the samples: uncoated and PTMC-coated Mg alloys in microfluidic system at different time
The histological evaluation of the vascular response to these implants was carried out qualitatively. Compare with the normal blood vessel and the PTMC coated samples, the uncoated Mg alloys could promote the acute thrombosis occurrence. There was lower coagulation reaction between PTMC coated samples and blood, tissue cells would like to live around the samples indicating better tissue compatibility existed.
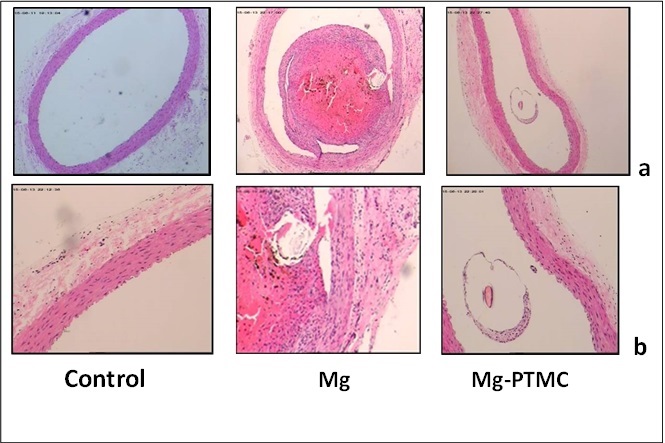
Fig3.The histological analysis of the coronary arteries after 15 days implantation: (HE staining, a:×40;b: ×100)
Conclusion: PTMC, as a kind of eroding coating has the potential to improve the the durability and biocompatibility of Mg-based stents.
This study was supported by the National Natural Science Foundation of China under Grant Nos. 81401522 and 81330031.
References:
[1] Erne P, Schier M, Resink TJ. The road to bioabsorbable stents: reaching clinical reality? Cardiovasc Intervent Radiol 2005;29:11–16
[2] Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials 2007;28: 1689–710
[3] Akira Mochizuki , Hideki Kaneda. Study on the blood compatibility and biodegradation properties of magnesium alloys. Materials Science and Engineering C 2015;47: 204–210
[4] Juan Wang , Yonghui He , Manfred F. Maitz ,etal, A surface-eroding poly(1,3-trimethylene carbonate) coating for fully biodegradable magnesium-based stent applications: Toward better biofunction, biodegradation and biocompatibility. Acta Biomaterialia 2013;9:8678–8689
[5] Dingchuan Xue, Yeoheung Yun, Zongqing Tan, In Vivo and In Vitro Degradation Behavior of Magnesium Alloys as Biomaterials. J. Mater. Sci. Technol., 2012; 28(3): 261-267