Introduction: Proper folding and assembling are key processes for biopolymers to exhibit their inherent biological activities. Artificial materials that are capable of manipulate these structures of biopolymer would have variety of applications in biomedical fields. We have designed ionic graft copolymers consisting of a polyion backbone and hydrophilic graft chains of dextran to engineer folding and assembling of biopolymers, such as DNA, having opposite ionic charges. Hydrophilic graft chains of polysaccharide guarantee soluble inter-polyelectrolyte complex formation with DNA. The copolymers significantly facilitate DNA assembly including DNA duplex, triplex and quadruplex formations, In this study, we examined the copolymer to enhance activity of deoxyribozymes, DNAzymes. We showed that the copolymers considerably increased catalytic efficacy of DNAzymes having ribonuclease activity, permitting us to refine DNAzyme-based DNA sensing.
Results and Discussion: Firstly, we investigated the effect of the copolymer PLL-g-Dex on ribonuclease activity of the 10-23 DNAzyme (Fig. 1) toward substrate I with 0.5 mM Mg2+ as a cofactor under multiple turnover conditions, [S]/[E] = 30[1]. Whereas in the absence of the copolymer kobs was 1.3×10-3 min-1, in the presence of the copolymer at an N/P ratio of 2, kobs was 3 fold greater.
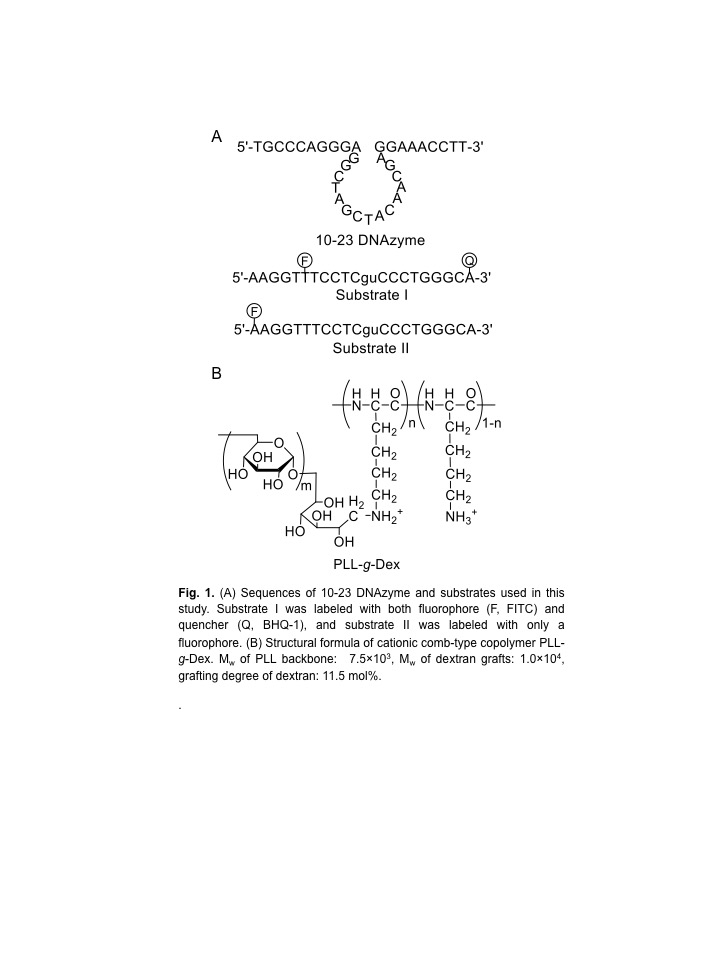
To estimate the role of the copolymer in the observed acceleration, the DNAzyme reactions were carried out under either single- or multiple-turnover conditions. In the reaction under the single-turnover condition no difference in cleavage rates was observed regardless of the presence of PLL-g-Dex. The results indicated that the copolymer did not significantly influence chemically cleavage activity of the DNAzyme. In contrast, under multiple-turnover reaction condition faster cleavage was observed in the presence of PLL-g-Dex, implying that the copolymer promoted turnover.
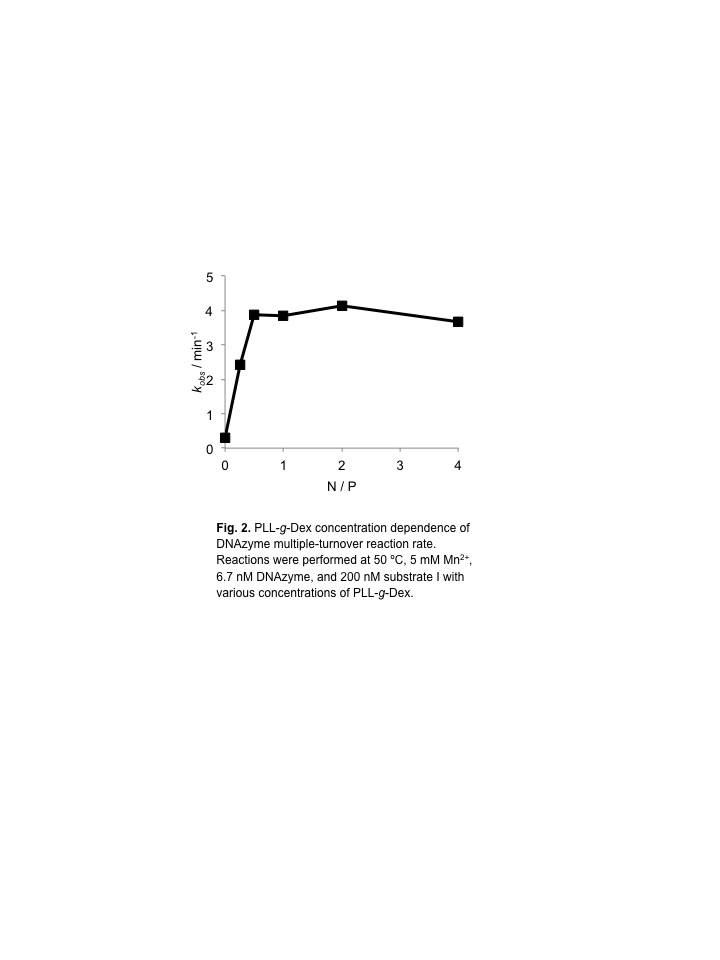
Next, we carried out the reaction with Mn2+ which is more active cofactor than Mg2+ either in the absence or presence of the copolymer (Fig. 2). The DNAzyme activity was enhanced with increasing concentration of the copolymer. The copolymer increased the cleavage rate more than 10 fold.
The activity of the copolymer was applied to DNAzyme-based nucleotide sensor, NMAzyme. The copolymer increased the MNAzyme reaction rate by 200 times, allowing target DNA detection at picomolar concentrations at physiological temperature[2]. The copolymer likely facilitated target-induced assembly of active MNAzyme complex. Notably, with the copolymer and the short-armed MNAzyme DNA was detected at sub-picomolar concentration at physiological temperature.
Although further improvement in the sensitivity will be required to enable utility in point-of-care diagnostic devices, use of the cationic copolymer will facilitate construction of simple and accurate assays using MNAzymes.
References:
[1] J. Gao, N. Shimada, A. Maruyama, MNAzyme-catalysed nucleic acid detection enhanced by a cationic copolymer, Biomater. Sci, 3, 716-720 (2015)
[2] J. Gao, N. Shimada, A. Maruyama, Enhancement of deoxyribozyme activity by cationic copolymers, Biomater. Sci., 3, 308-316 (2015)