Purpose: A study was conducted to assess the effectiveness of various sterilization and disinfection treatment processes in depyrogenation of bovine collagen. Pyrogens are non-living fever producing agents not rendered innocuous by bactericides, moldicides, and gases[1]. In order to eliminate pyrogens in medical devices, novel methods which do not disrupt the structural stability of the device during the treatment process are needed[2]. In this study, collagen fibers/particulate/powder were subjected vapor hydrogen peroxide (VHP), chlorine dioxide (ClO2), dehydrothermal treatment (DHT), nitrogen dioxide (NO2), and liquid hydrogen peroxide (LHP) treatment processes, individually. Following exposure, the collagen underwent Limulus Amebocyte Lysate (LAL) testing to determine residual pyrogen levels[3]. To assess the structural integrity following exposure, differential scanning calorimetry (DSC) was implemented to determine the dissociation temperatures of the collagen[4]. We showed that VHP, DHT, and ClO2 processes resulted in a significant reduction in pyrogen levels (>80%). Compared to the unprocessed collagen, the samples’ transition temperatures were not significantly different for the VHP, ClO2, and 3 hour DHT processes, suggesting that no significant change in structural properties of collagen occurred as a result of these treatment processes.
Methods: Purified bovine hide collagen was exposed to one of the following processes to evaluate depyrogenation effectiveness: VHP, ClO2, NO2, DHT, or LHP treatment. VHP was performed at Steris (4 hour, 800ppm), ClO2 at ClorDiSys (720ppm/hour dosage), NO2 at Noxilizer (10mg/L NO2, 4 pulse, 10 minute dwell), DHT at NovaBone using the MillRock RD85 series Lyophilizer (3, 6, 12, and 24 hour cycles), and LHP at NovaBone (2 hour soak in 3% solution). After processing, samples were sent to Nelson Laboratories (Salt Lake City, UT) for LAL testing (0.005 EU/mL sensitivity, 1:10 dilution, 60 minute extraction at 37-40⁰C). For DSC testing, 25-40mg samples of hydrated collagen were placed in sealed aluminum crucibles. The crucibles were placed in the Shimadzu DSC-60 and heated from 25°C to 80°C at a rate of 5°C per minute. Tangential analysis was used to assess transition temperature.
Results: A minimum of n=3 samples per treatment group was assessed for LAL testing and transition temperatures. Results of LAL testing and transition temperature testing for each processing condition are summarized in Table 1; DSC testing was performed only on samples that showed a statistically significant reduction in pyrogen levels (p<0.05). Additionally, DSC testing was not performed on 6 and 12 DHT samples because these sample did not perform as desired in a separate functionality study.
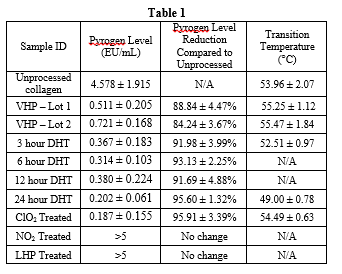
One-way ANOVA with Dunnett’s Tests were run to determine significant differences in pyrogen levels as determined with LAL testing (Figure 1) and to determine significant differences in transition temperatures (Figure 2). LAL test results indicate that all processing conditions exhibited significantly lower (p<0.05) pyrogen levels as compared to the unprocessed collagen with the exception of NO2 and LHP treated samples. Transition temperature results indicate that there was no significant difference in the transition temperature of the processed collagen samples compared to the unprocessed collagen for VHP, 3 hour DHT, and ClO2 conditions; 24 hour DHT exhibited a significantly lower transition temperature (p<0.05).
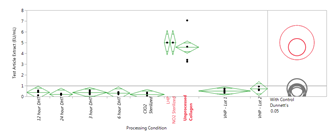
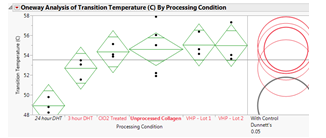
Conclusions: Exposing 100% purified bovine hide collagen to VHP, DHT, and ClO2 cycles results in a significant reduction in pyrogen levels. Additionally, transition temperature assessment using DSC shows that VHP, 3 hour DHT, and ClO2 treated collagen samples are not significantly structurally different as compared to unprocessed collagen. A next step in the study will be to further investigate the collagen structure after each treatment process using circular dichroism.
References:
[1] Mamerto C. Method for preparing pyrogen free collagen. 1980
[2] Vetten, Melissa A., et al. "Challenges facing sterilization and depyrogenation of nanoparticles: Effects on structural stability and biomedical applications."Nanomedicine: Nanotechnology, Biology and Medicine 10.7 (2014): 1391-1399
[3] United States Food and Drug Administration, “Guidance for Industry. Pyrogen and Endotoxins Testing: Questions and Answers,” June 2012. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm314718.htm
[4] Skrzyński, S., A. Sionkowska, and A. Marciniak. "DSC study of collagen in disc disease." Journal of Biophysics 2009 (2010)