Bone surface microenvironment mimicked PLA scaffolds for osteogenic stem cell differentiation
-
1
Bogazici University, Institute of Biomedical Engineering, Türkiye
Introduction: The importance and advantages of bone tissue engineering products are increasing because of the disadvantages of autografts, allografts and zenografts such as finding suitable donors, rejection of tissue by immune system or pathogen carrying properties which are commonly using for the treatment of bone defects[1]. Changing the surface properties of scaffolds such as surface stiffness, chemical and biochemical components, surface roughness and topography may influence and effect the cell-surface, cell-scaffold interface characteristics and cellular behavior and mechanism of osteoblasts, stem cells and as well as other cell types[2].
Materials and Method: Bovine femur bone surface was mimicked by using a biodegrable polymer, Poly (L-Lactic acid) on the PDMS mold to obtain bone surface mimicked scaffolds. After the optimization of bone surface mimicking conditions, bone morphogenic protein 2 (BMP-2) was loaded on bone surface mimicked PLA scaffolds and release profile was examined at in-vitro conditions. Bone surface mimicked PLA scaffolds were modified with hydroxyapatite (HA) or collagen (Col I) in order to construct these scaffolds similar to the bone`s natural microenvironment. Bone surface mimicked, BMP-2 loaded, HA or Col I modified PLA scaffolds were characterized by water contact angle measurements, Scanning Electron Microscope (SEM), Atomic Force Microscope (AFM) and degradation tests. These scaffolds were used for the controlled and directed differentiation of adipose derived stem cells and bone tissue formation in in-vitro conditions.
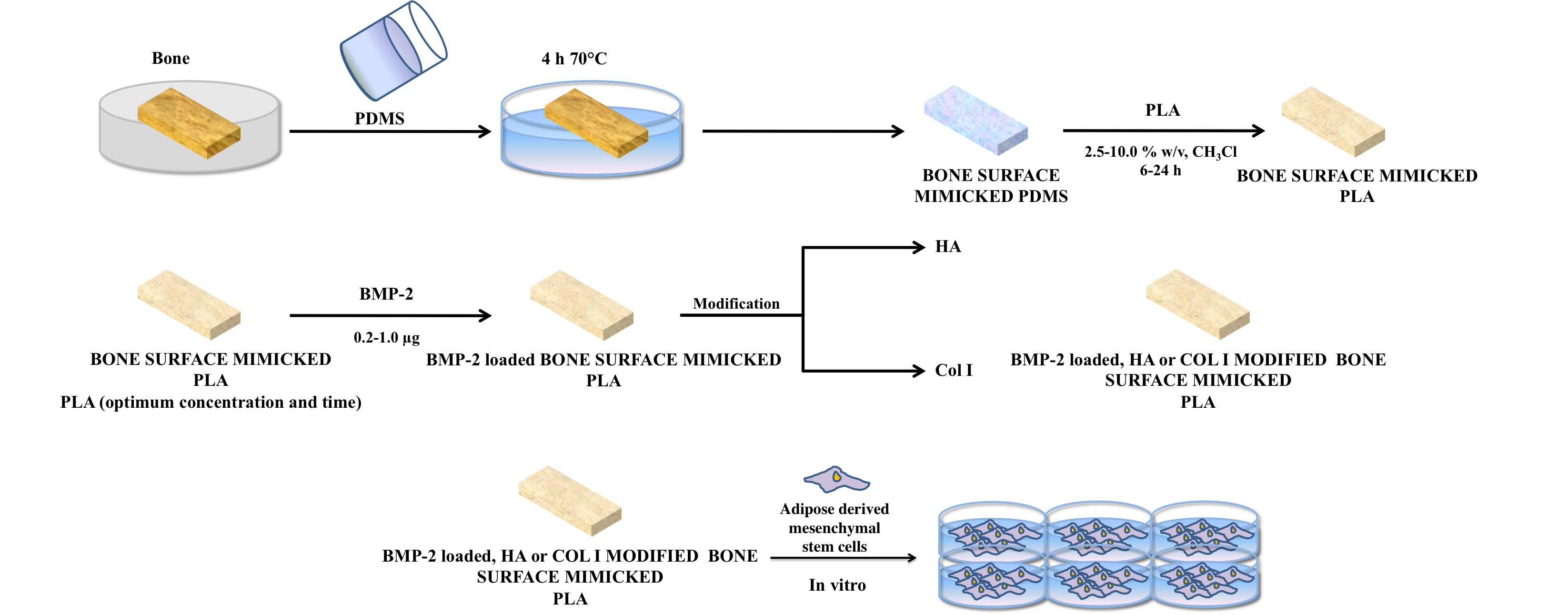
Results: In the study for the first time bone surface mimicked scaffolds were and characterized and the effect of these scaffolds in in-vitro conditions to the differentiation of adipose derived mesenchymal stem cells were investigated.
Discussion: Hydroxyapatite (HA) or collagen (Col I) modified bone surface mimicked and PLA scaffolds have shown better osteogenic differentiation profile of Adipose derived mesenchymal stem cells then plain PLA scaffolds and control group.
Conclusion: Bone surface microenvironment were physically, chemically and biochemically prepared by using PLA scaffolds. These scaffolds could be used for the controlled and directed differentiation of adipose derived stem cells to osteoblasts in in-vitro conditions.
This work is supported by TUBITAK Project under Grant No. 113S730 and Boğaziçi University Research Fund by Grant Number No. 6701.
References:
[1] SULTANA N., Biodegradable Polymer-Based Scaffolds for Bone Tissue Engineering, Springer Briefs in Applied Sciences and Technology, 1-62, 2013.
[2] KUMBAR S.G., Kofron M.D., Nair L.S., Laurencin C., Cell Behavior Toward Nanostructured Surfaces, Biomedical Nanostructures,263. 2008.
Keywords:
stem cell,
Biodegradable material,
biomimetic culture,
surface topolography
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomimetic materials
Citation:
Ozcolak
B,
Perver
D,
Ozkara Yavuz
S,
Jandt
KD and
Garipcan
B
(2016). Bone surface microenvironment mimicked PLA scaffolds for osteogenic stem cell differentiation.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01112
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Birgun Ozcolak, Bogazici University, Institute of Biomedical Engineering, Istanbul, Türkiye, mervebirgunozcolak@gmail.com
Dr. Dilara Perver, Bogazici University, Institute of Biomedical Engineering, Istanbul, Türkiye, dilaraperver@gmail.com
Dr. Serpil Ozkara Yavuz, Bogazici University, Institute of Biomedical Engineering, Istanbul, Türkiye, soyavuz@anadolu.edu.tr
Dr. Klaus D Jandt, Bogazici University, Institute of Biomedical Engineering, Istanbul, Türkiye, k.jandt@uni-jena.de
Dr. Bora Garipcan, Bogazici University, Institute of Biomedical Engineering, Istanbul, Türkiye, Email1