Thermally-modulated stem cell purification using thermoresponsive ionic copolymer brush
-
1
Tokyo Women’s Medical University, Institute of Advanced Biomedical Engineering and Science, Japan
-
2
Waseda University, Department of Life Science and Medical Bioscience, Japan
Introduction: In regenerative medicine, an effective cell separation method that can provide adequate purity, yield, and function have been needed for effective fabrication of transplantable tissues. As new cell separation tool, we prepared thermoresponsive cationic copolymer brush, poly(N-isopropylacrylamide(IPAAm)-co-N,N-dimethylaminopropyl acrylamide(DMAPAAm)-co-N-tert-butylacrylamide (tBAAm)) brush grafted surfaces through surface-initiated atom transfer radical polymerization (ATRP).
Adhesion and detachment behaviour of human bone marrow mesenchymal stem cell (hbmMSC) and other human bone marrow derived cells on the ionic copolymer brush was observed for investigating the possibility of the prepared copolymer brush as a stem cell purifying materials.
Experimental: An ATRP initiator, 2-(m/p-chloromethylphenyl) ethyltrimethoxysilane, was modified on glass coverslips (Fig. 1). Copolymerization of IPAAm (75mol%), DMAPAAm(5mol%), and tBAAm(20mol%) was performed through surface-initiated ATRP for 16 h at 25°C using CuCl/Me6TREN as a catalyst and 2-propanol as reaction solvent. In the polymerization, a-chloro-p-xylene was added as a free ATRP-initiator and brush length was estimated using the synthesized copolymer in the reaction solution. Cell adhesion and detachment behaviour of hbmMSC and other human bone marrow derived cells were observed at 37 and 20°C.
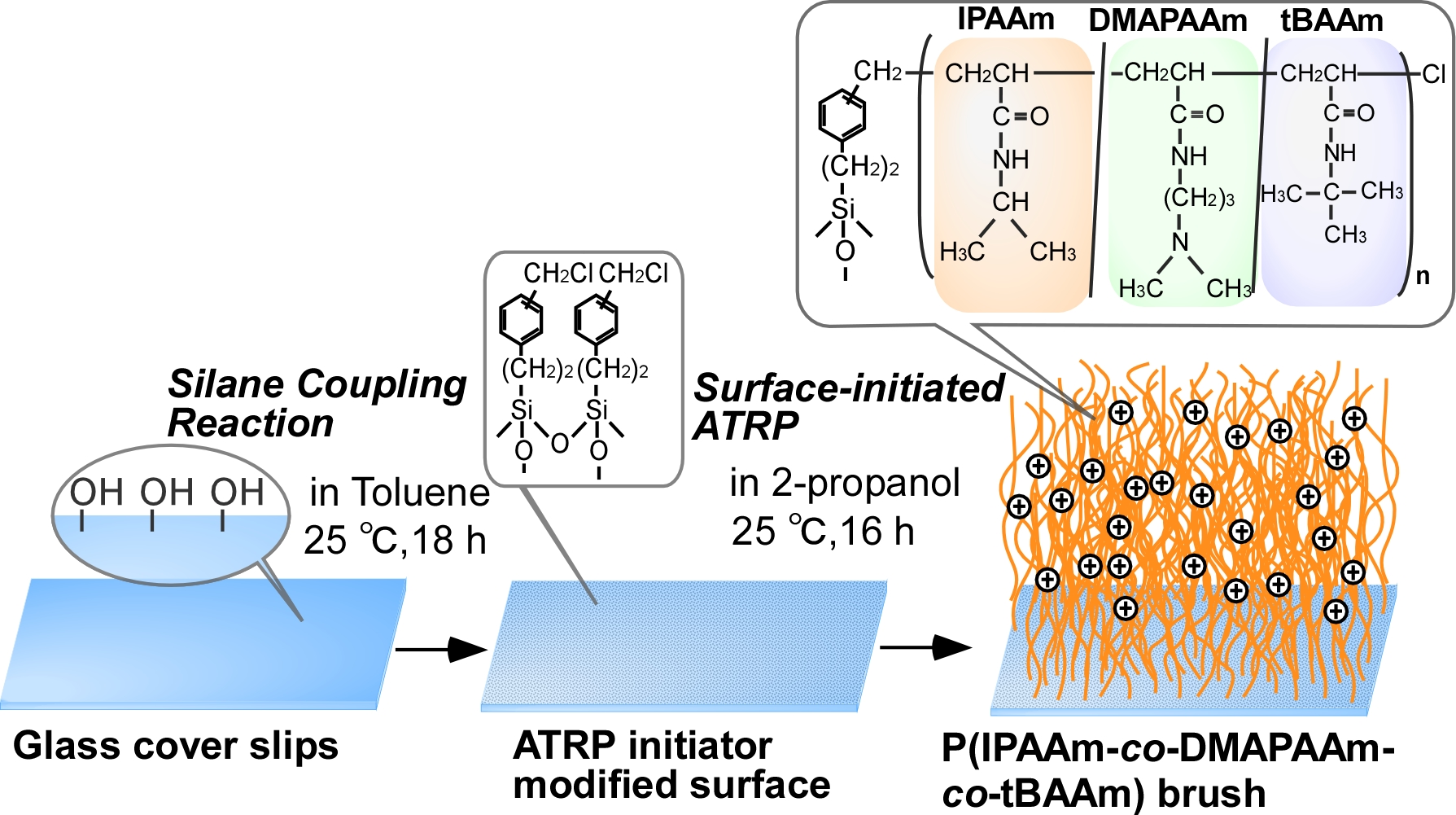
Results and Discussion: Prepared thermoresponsive cationic copolymer brush was characterized by ATR/FT-IR, GPC measurement, and zeta potential measurement. As result, amount of grafted copolymer and graft density were 1.51 mg/cm2 and 1.04 chains/nm2, respectively. Zeta potential of copolymer brush was 19.8 mV. These result indicated that dense thermoresponsive cationic copolymer brush was formed on glass substrate. Cells adhesion and detachment properties on copolymer brush were observed using hbmMSC and other bone marrow derived cells. hbmMSC adhered on the cationic copolymer brush at 37 °C, while other bone marrow derived cells did not. With reducing temperature to 20 °C, adhered hbmMSC detached from cationic copolymer brush. Using this properties, purification of hbmMSC was performed simply by changing temperature. Mixture of human bone marrow derived cells were seeded on cationic copolymer brush. Only hbmMSC adhered on cationic copolymer brush at 37°C. Then, temperature was reduced to 20 °C, and purified hbmMSC was successfully recovered from the copolymer brush (Fig.2).
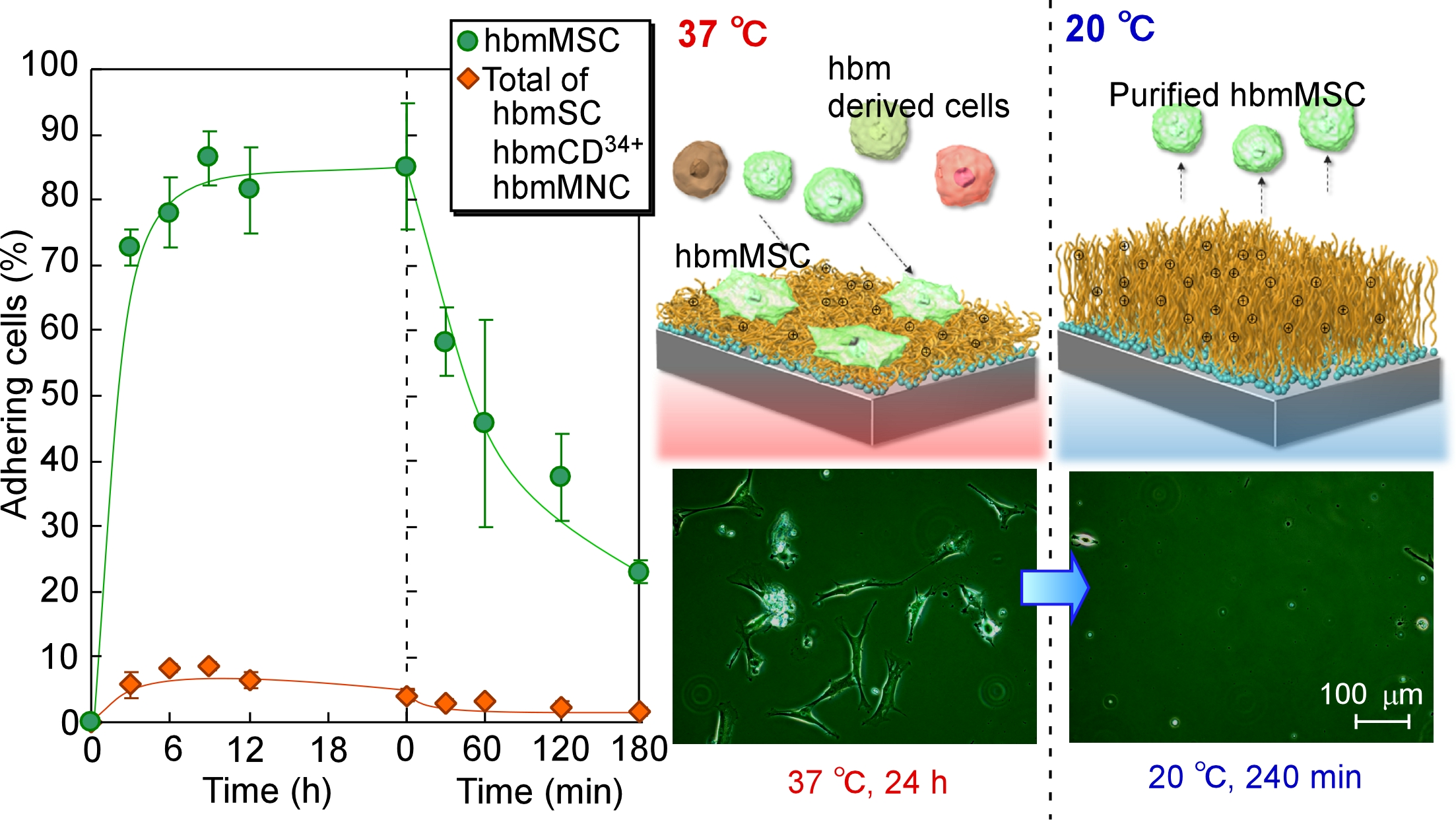
The result indicated that prepared thermoresponsive cationic copolymer brush would be useful for purification of hbmMSC from other cells.
Conclusion: Thermoresponsive cationic copolymer brush prepared through surface-initiated ATRP can selectively adhered and detach hbmMSC by external temperature change. Thus, the thermoresponsive cationic copolymer brush would be useful for purification of hbmMSC simply by changing temperature.
Keywords:
Surface modification,
polymer brush,
stimuli-response,
Cell modulation
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
General Session Oral
Topic:
Interfacial phenomena
Citation:
Nagase
K,
Hatakeyama
Y,
Shimizu
T,
Matsuura
K,
Yamato
M,
Takeda
N and
Okano
T
(2016). Thermally-modulated stem cell purification using thermoresponsive ionic copolymer brush.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01137
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Kenichi Nagase, Tokyo Women’s Medical University, Institute of Advanced Biomedical Engineering and Science, Tokyo, Japan, Email1
Dr. Yuri Hatakeyama, Waseda University, Department of Life Science and Medical Bioscience, Tokyo, Japan, Email2
Dr. Tatsuya Shimizu, Tokyo Women’s Medical University, Institute of Advanced Biomedical Engineering and Science, Tokyo, Japan, Email3
Dr. Katsuhisa Matsuura, Tokyo Women’s Medical University, Institute of Advanced Biomedical Engineering and Science, Tokyo, Japan, Email4
Dr. Naoya Takeda, Waseda University, Department of Life Science and Medical Bioscience, Tokyo, Japan, Email5
Dr. Teruo Okano, Tokyo Women’s Medical University, Institute of Advanced Biomedical Engineering and Science, Tokyo, Japan, Email6