Introduction: The control of stem cell behaviour on biomaterial surfaces is the key to a broad range of biomedical applications. The specific surface properties of biomaterials such as nanotopography and chemistry can profoundly influence cell morphology, proliferation, differentiation and even cell-cell communication. Nanotopography alone has been shown that can stimulate osteogenesis of mesenchymal stem cells (MSCs)[1]. It provides a more controllable way to direct stem cells than chemical induction. Recently, we have established a group of elaborate surfaces to display ordered topographies with tuneable chemistry called self-assembled monolayer binary colloidal crystals (BCCs), and introduced them as substrates for cell culture (Fig. 1)[2].

Figure 1. Schematic illustration of the fabrication of binary colloidal crystals (BCCs) for use in cell culture. (A) The BCCs were fabricated using EICSA. (B) The BCCs were stabilized by melting the small particles. (C) hMSCs formed colonies on BCCs after chondrogenic differentiation.
Materials and Methods: The BCCs were fabricated using evaporation induced colloidal self-assembly (EICSA)[2],[3]. We screened over 100 combinations of binary colloidal mixtures and only selected four BCCs with large surface coverage with different sizes and surface chemistries for stem cell culture (i.e. 5SiPMMA, 5SiPS, 2SiPMMA, and 2SiPS). After heat stabilisation, surfaces were characterised using atomic force microscopy (AFM), scanning electron microscopy (SEM), and water contact angle goniometry. Cellular responses of hMSCs and pluripotent stem cells on the BCCs were investigated in terms of attachment, growth, and differentiation. Fluorescence staining, qPCR, and FACS using specific markers at different time points were used to study the cell-BCC interactions.
Results: Four selected BCCs have different structures and chemistries resulting in complex surface properties i.e. different surface roughnesses and wettability (Fig. 2). In general, stem cells don’t like the surface properties of BCCs and have a smaller spreading area compared with controls. This change affects a series of subsequent cell behaviours such as differentiation. Chondrogenesis of hMSCs on BCCs was enhanced after 1 week culture compared with Si control (Fig. 3).
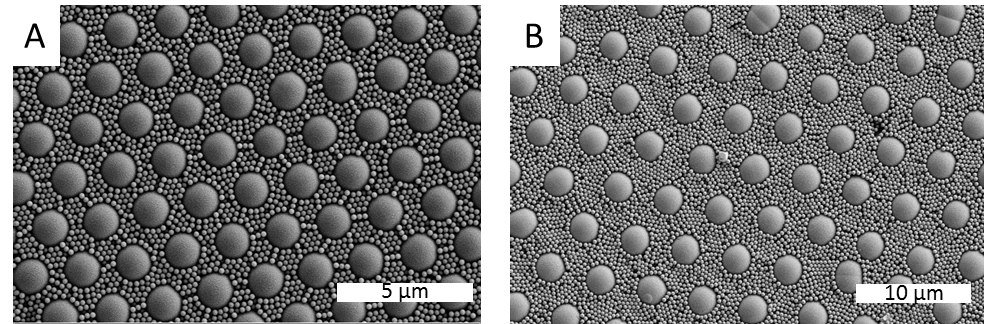
Figure 2. SEM images of (A) 2SiPS and (B) 5SiPMMA. A highly ordered hexagonal closed-packed structure can be found on the surfaces with different particle spacing which leads a different roughness and wettability.

Figure 3. (A) Bright-field and (B) fluorescence image of chondrogenic differentiated hMSCs after 1 week on 5SiPMMA. Cells formed colonies on BCCs, but not on Si controls.
Discussion: The surface properties of the BCCs are highly complex and their properties dominate stem cell behaviour, where in general BCCs can modulate cell attachment and influence stem cell differentiation. Furthermore, surface modification using selective grafting on BCCs is straightforward due to the inherent functionality of the particles used, while it is quite difficult on other surfaces. Results will be presented on how that step improves cell behaviour further.
Conclusion: This study established a new platform for cell culture and demonstrated that the BCCs are promising in controlling stem cell behaviour. The findings of cell-surface interactions could also benefit the development of biomaterials and tissue engineering.
The Scientific Industrial Endowment Fund (SIEF) and The Discovery Early Career Researcher Award (DECRA) are acknowledged for providing Postdoctoral Research Fellowship for PYW. PYW acknowledges Wendy Zeng for the helping of qPCR. This work was performed in part at both the Biointerface Engineering Hub at Swinburne and MCN as part of the Victorian Node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australia’s researchers.
References:
[1] Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 2007;6:997-1003.
[2] Wang PY, Pingle H, Koegler P, Thissen H, Kingshott P. Self-assembled binary colloidal crystal monolayers as cell culture substrates. J Mater Chem B 2015;3:2545-52.
[3] Singh G, Pillai S, Arpanaei A, Kingshott P. Multicomponent colloidal crystals that are tunable over large areas. Soft Matter 2011;7:3290-4.