Introduction: Nitric oxide (NO) is primarily released by the endothelium cells with a flux of 0.5-4 x 10-10 mol cm-2 min-1 and is a potent antithrombotic and antimicrobial agent[1]. A wide variety of NO donors incorporated into various polymers have been studied as NO releasing materials. However, problems associated with rapid NO donor leaching and donor instability exist have prevented the commercial applications of NO releasing polymers. Herein, we report our efforts to prepare S-nitroso-N-acetyl-penicillamine (SNAP) - impregnated CarboSil 20 80A polymer via solvent swelling methods[2], which exhibit long-term NO release and the ability to reduce thrombus formation and bacterial adhesion when implanted in long-term freely moving animals as compared to the control catheters.
Materials and Methods: CarboSil polymer films with various wt% SNAP were prepared by swelling the polymer films in SNAP solutions with different concentration for 2 h at room temperature. Films were then taken out from the swelling chamber and dried under ambient environment. Powder X-ray diffraction was utilized as the solid-state analysis to investigate the SNAP impregnated CarboSil films. Plain CarboSil catheters were prepared by dipcoating on stainless steel mandrels[3] and then impregnated with the SNAP swelling solution. NO release from the catheters was monitored via a chemiluminescence NO analyzer at 37°C. Finally, 15 wt% SNAP-impregnated CarboSil catheters were prepared, sterilized (by ethylene oxide (EtO)) and implanted in veins of freely moving rabbits (10 d) and sheep (21 d) to evaluate the antithrombotic and antimicrobial efficacies of the material. Thrombus formation area on the catheter surface was quantified using a 2D representation imaging software (NIH Image J) after the removal of the catheters. The viable bacteria number on both catheter surfaces was determined by homogenizing the catheter surface, serial dilution and plate counting.
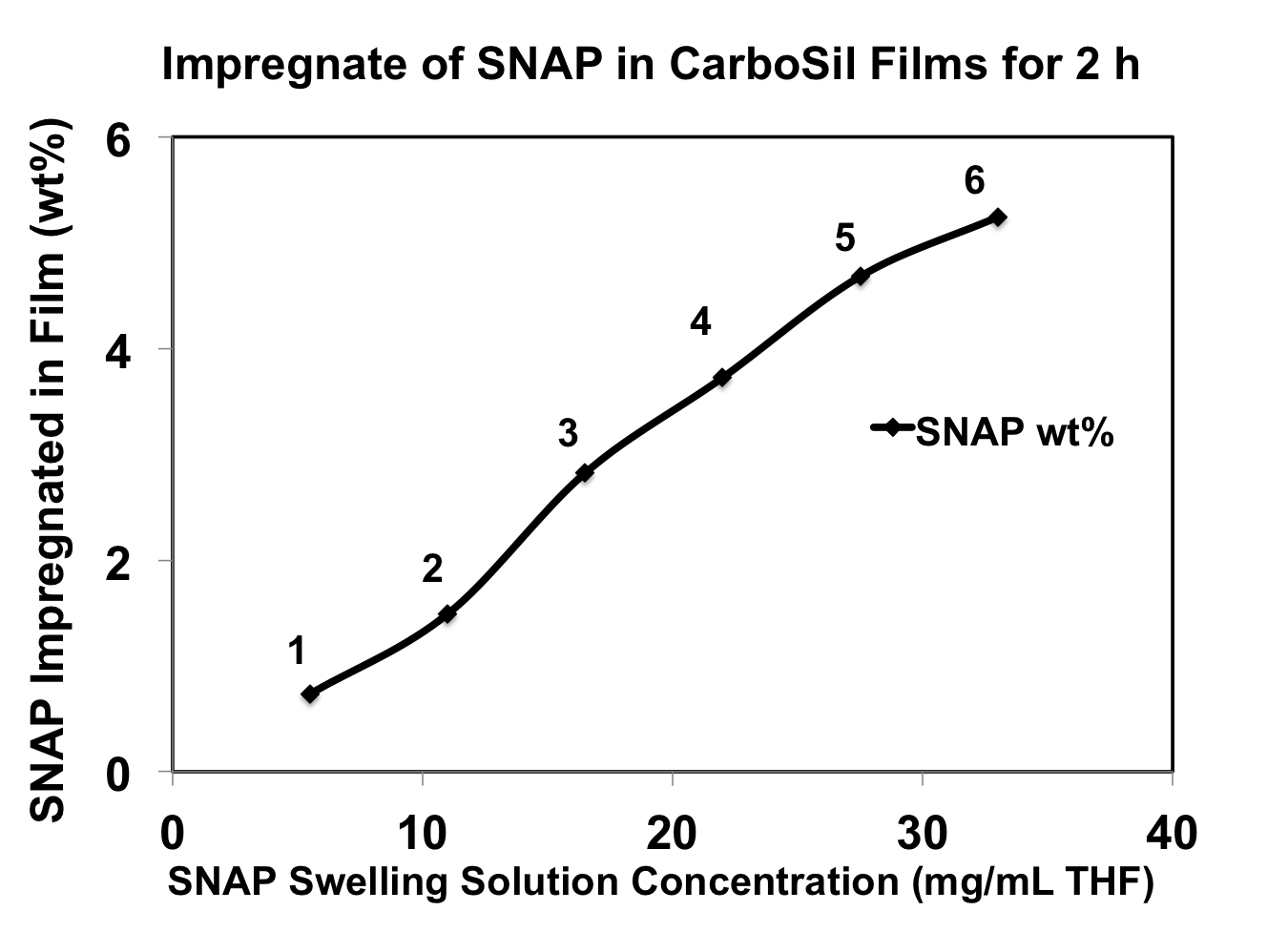
Results and Discussion: Fig. 1 illustrates that when the swelling time is constant (2 h), the amount of SNAP incorporated in films is concentration dependent of the SNAP swelling solution.
PXRD characterization of SNAP-impregnated CarboSil films demonstrates that the characteristic film diffraction patterns are convolutions of blank Carbosil and the crystalline SNAP powder patterns. And only when SNAP concentration is higher than the solubility threshold of the CarboSil polymer (3-4 wt%), then it exists in an orthorhombic crystal form within the bulk of the polymer (see Fig. 2), and stabilized by intermolecular H-bonds. The 15 wt% SNAP impregnated CarboSil catheters has excellent stability during EtO sterilization (55 °C, 12h), where 97.8% of the initial SNAP remained in the polymer after the process. The catheters are shown to release NO >0.5 flux (physiological level) for 3 weeks. Catheters implanted in rabbits and sheep both demonstrate significantly reduction of thrombus formation and bacterial adhesion on the surfaces of the SNAP catheters when compared to the controls.
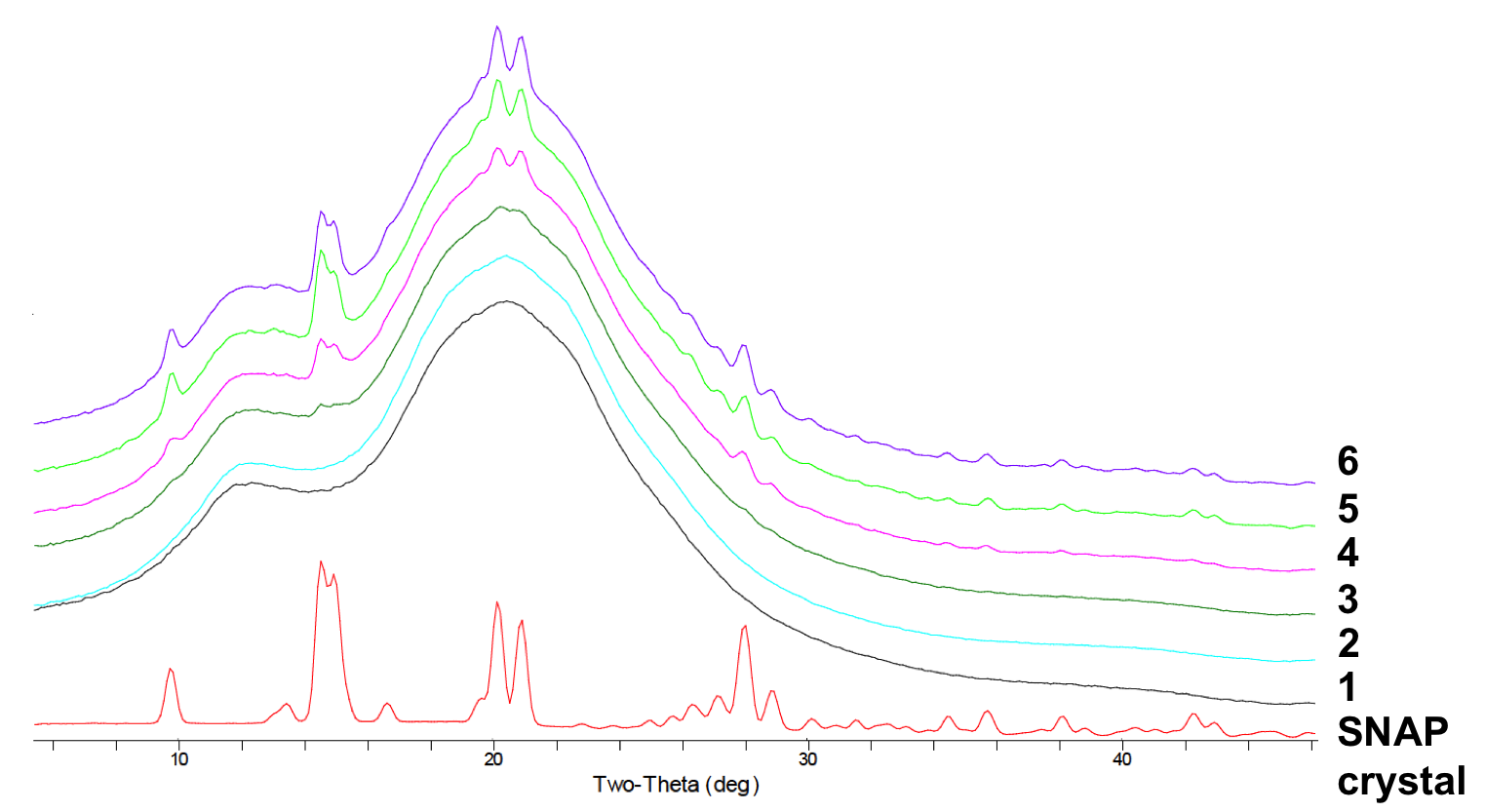
Conclusion: S-nitroso-N-acetylpenicillamine (SNAP) was successfully swelled/impregnated into existing biomedical grade polymers, CarboSil 20 80A, which is an excellent matrix to act as reservoir for SNAP. The amount of SNAP in polymer can be controlled by varying the swelling solution concentration. PXRD characterization suggests that when the SNAP concentration exceeds the solubility limit of CarboSil (3-4 wt%), SNAP exists as crystals in the polymer, which are stabilized by intermolecular H-bonds. The SNAP impregnated CarboSil catheters demonstrates the ability to reduce surface thrombus formation and bacteria adhesion in long-term animal implantations. Since no extrusion process is required for the incorporation of the NO donor into the pre-made catheter walls, this method (with little or no chemical degradation of the SNAP) have a great potential to be applied to a wide variety of existing polymeric biomedical devices that are in need of providing antithrombotic and antimicrobial surfaces.
NIH fundings (EB-000783, R41-DK101206-01and GM106180) and the gift of polymers from DSM, CA
References:
[1] Brisbois et al., Biomaterials 2013, 34 (28), 6957–6966.
[2] Colletta et al., ACS Biomater. Sci. Eng. 2015, 1, 416–424.
[3] Brisbois et al., J. Mater. Chem. B 2015, 3, 1639–1645.