Introduction: RNA interference (RNAi) is a powerful tool for regulating cellular gene expression with numerous therapeutic targets. However, translation is impeded by off-target effects from systemic administration, rapid biodegradation of unprotected RNA, limited therapeutic windows, and low efficiency in vivo. For these reasons, there is need for an injectable, minimally invasive delivery system for local and sustained RNAi therapy. Towards this, our lab recently modified hyaluronic acid (HA) with β-cyclodextrin (CD, host) or adamantane (Ad, guest) to form injectable gels assembled through guest-host interactions that allow for controlled biomolecule delivery [1]. Here, we designed a series of guest-host modified gels with varied polymer charge to optimize these materials for sustained and effective RNAi delivery.
Materials and Methods: Polymer synthesis: HA, 8-arm polyethylene glycol (PEG), and polyethylenimine (PEI) were modified with either Ad or CD and mixed for gel formation. Particle sizing: Dynamic light scattering (DLS) measurements were performed on a Zetasizer Nano from Malvern Instruments. Cell Culture and Transfection: NIH/3T3 cells were plated at 1x105/mL prior to transfection. PEI/siRNA complexes (25 nM) were formed in DMEM and added to cells. Gel siRNA Encapsulation: 100 µL Gels were formulated in PBS containing 13.3 µg Cy3-siRNA. DMEM was collected and replaced over two weeks and added to cells. Uptake of Cy3-siRNA was assessed by flow cytometry.
Results and Discussion: Polymers with varied charge (HA: anionic, PEI: cationic, PEG: neutral) were synthesized that formed gels via the guest-host interaction of Ad and CD. The gels were shear-thinning and self-healing to permit injection. However, when PEI/siRNA polyplexes were mixed with unmodified HA and PEG, HA led to aggregates (Fig 1A) with little transfection (Fig 1B), whereas PEG did not aggregate polyplexes and led to high transfection.
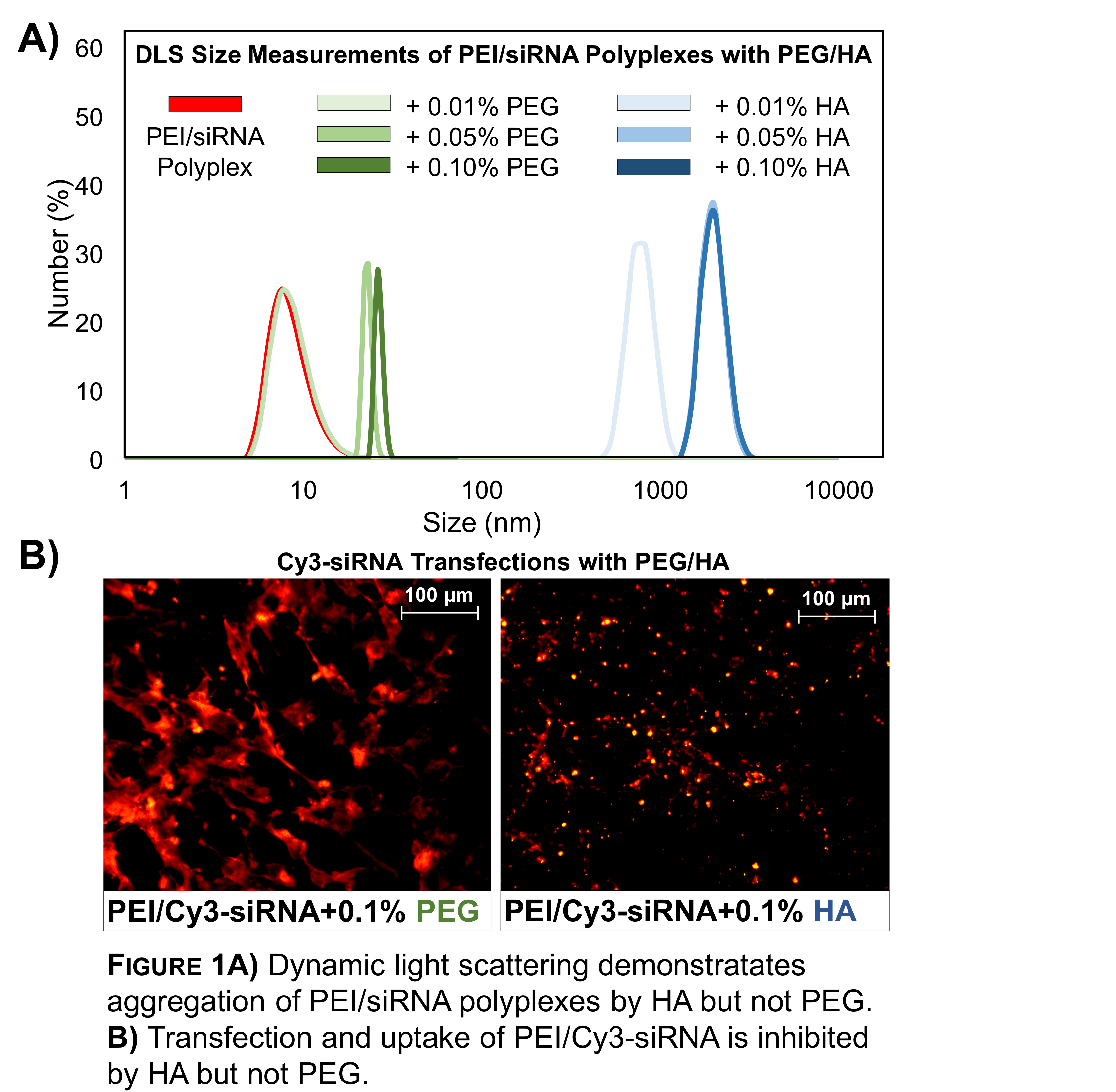
Moreover, CD-PEI/Ad-PEG nanocomplexes have previously been successful as transfection agents [2]. Thus, Ad-PEG and CD-PEI were formed into injectable gels and explored for long-term siRNA release. Cy3-siRNA was encapsulated and released (Fig 2A). The releasate was able to transfect 3T3 fibroblasts, as measured by fluorescent imaging and flow cytometry for over two weeks (Fig 2B). In this system, PEI acts as the transfection agent to complex RNA, as well as a component of the gel through guest-host interaction, enabling long-term bioactive release. Ongoing work is to further optimize gel formulations and perform in vivo assessment.
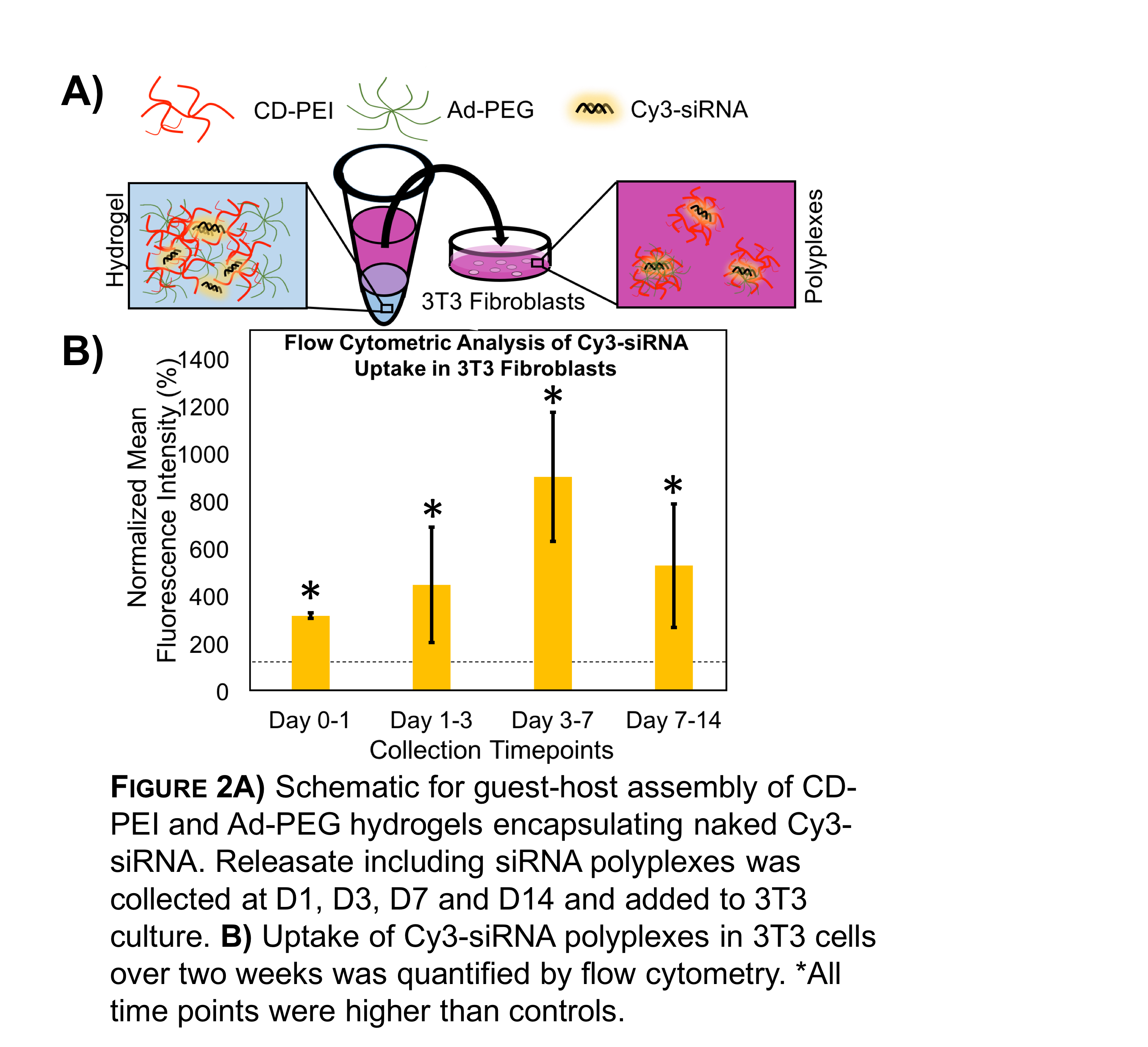
Conclusion: Here, we demonstrate the importance of polymer backbone charge in designing gels for RNAi delivery. Recognizing the importance of cationic charge, we developed a guest-host assembled CD-PEI/Ad-PEG gel that encapsulated naked siRNA and allowed for sustained release and uptake of active siRNA polyplexes over two weeks.
Financial support provided by NIH Grant R01HL111090 and Established Investigator Award (J.A.B.) from the American Heart Association.
References:
[1] Rodell CB, et al. Biomacromolecules. 2013.
[2] Pun SH, et al. Bioconjugate Chemistry. 2004.