The lack of specificity in traditional chemotherapeutic administration typically leads to significant dose-limiting toxicities and requires patients to wait for long periods between treatments. During this time, cancer cells have an opportunity to recover from the treatment and develop multi-drug resistance[1]. Our work holds promise to improve treatment specificity through the use of intelligent nanoscale hydrogels (nanogels) to localize the chemotherapeutic agents (CA) at targeted disease sites via the enhanced permeation and retention effect, ultimately limiting the toxicity to healthy tissues. Further, the nanogel molecular architecture can be tailored to carry a variety of cargos with widely varying physicochemical properties, promote cellular uptake, and release the cargo only in response to the intracellular environment. Nanoparticle-mediated combination therapy offers many advantages including the ability to signal different pathways in the cancer cells, maximize the therapeutic efficacy against specific targets, target different phases of the cell cycle, and overcome efflux-driven mechanisms of resistance[1]. Further, it allows PK/PD to be dictated by the in vivo distribution and cellular uptake of the nanogels rather than the physicochemical properties of the free CAs, ensuring optimal synergistic ratios are delivered to the cytosol[2].
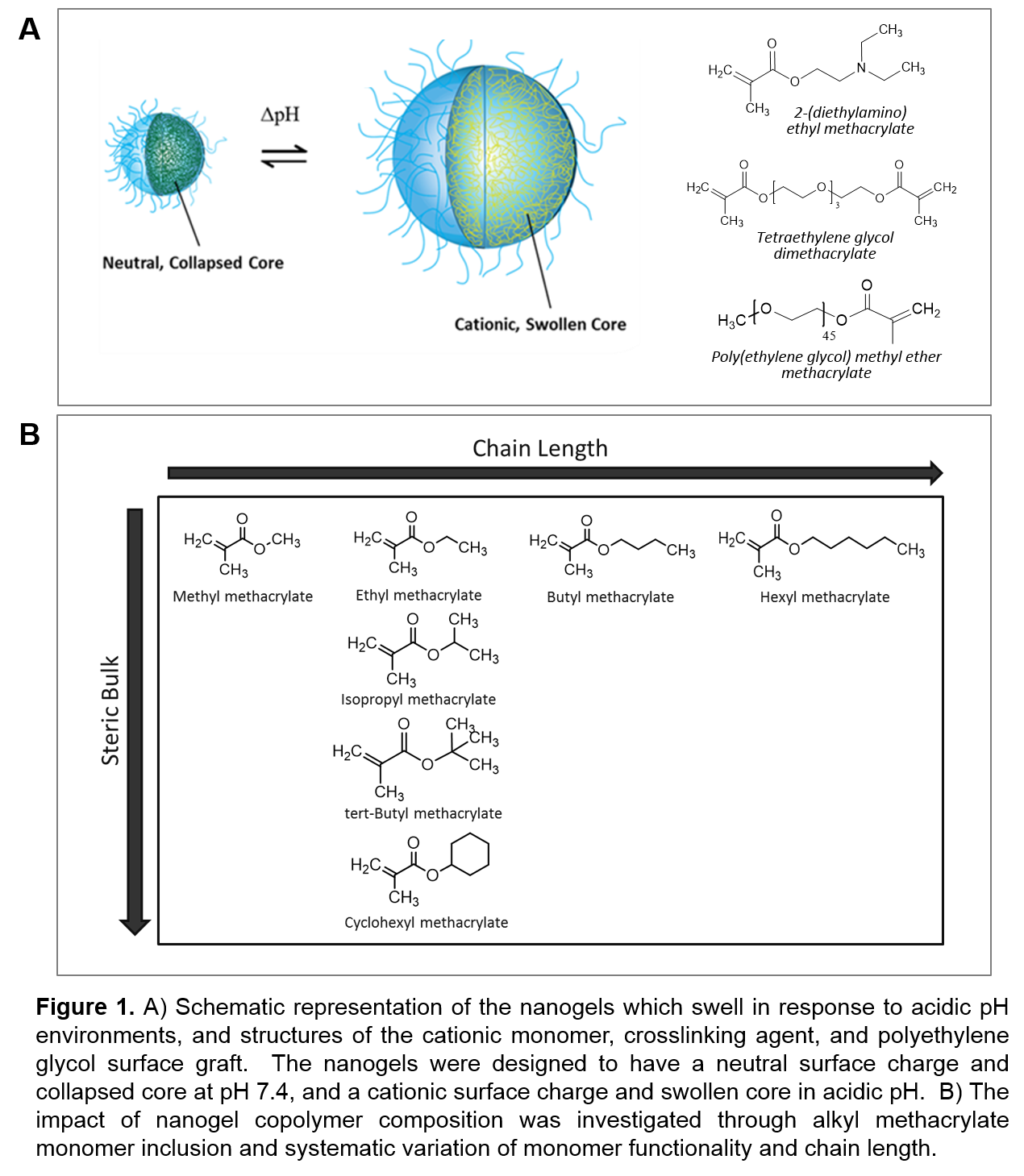
As shown in Fig. 1A, the nanogels are comprised of: 1) a cationic monomer 2-(diethylamino)ethyl methacrylate that imparts the pH-response by ionization of amine pendant groups, 2) a tetraethylene glycol dimethacrylate crosslinker to improve CA retention, 3) an alkyl methacrylate monomer to improve CA-polymer interactions, 4) surface-grafted poly(ethylene glycol) methacrylate to impart serum stability. Nanogels were synthesized using a UV-initiated oil-in-water emulsion polymerization[3] with a 2.5 mol% crosslinking density. The impact of alkyl methacrylate monomer inclusion was investigated through systematic variation of monomer functionality and chain length (methyl methacrylate, ethyl methacrylate, isopropyl methacrylate, tert-butyl methacrylate, cyclohexyl methacrylate, butyl methacrylate, and hexyl methacrylate as shown in Fig. 1B). The physical properties of the resulting nanogels were compared using dynamic light scattering, zeta potential, titration, pyrene fluorescence, and red blood cell hemolysis as a function of pH to elicit the influence of polymer composition on swelling ratio, surface charge, pKa, relative hydrophobicity and hydrophile-hydrophobe phase transition, and erythrocyte membrane disruption capability. The therapeutic delivery potential was analyzed using hydrophobic (paclitaxel) and hydrophilic (carboplatin) chemotherapeutic agents. Nanogels were loaded by imbibition at pH 4.0, and the release kinetics were studied by incubating loaded nanogels at pH 7.4 for 2 hr followed by pH 5.5 for 24 hr.
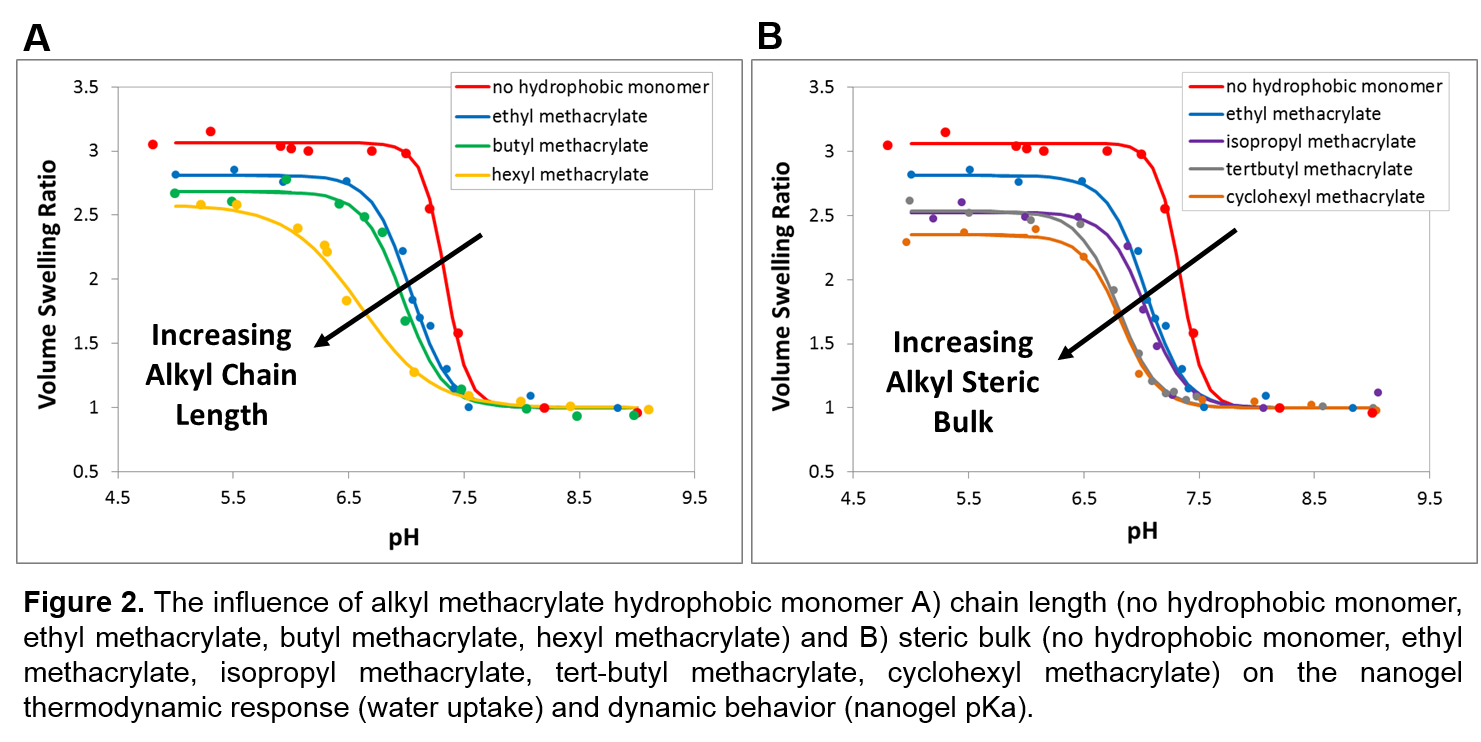
The nanogels resulted in well-defined and controllable particle size, morphology, and composition. We demonstrated the tunability of our multicomponent nanogel systems to entrap varied molecular cargos, and showed that the molecular architecture can be rationally designed to respond intelligently to different environments. As shown in Fig. 2a and 2b, inclusion of a hydrophobic monomer significantly altered the resulting nanogel physical properties. Varying both the chain length and steric bulk allowed for precise control over the thermodynamic response (relative swelling ratio), dynamic behavior (nanogel pKa and membrane disruption potential), and CA-polymer interaction (therapeutic delivery potential). Nanogels synthesized with hexyl methacrylate exhibited favorable behavior for intracellular delivery and demonstrated an increase in the therapeutic delivery potential of both hydrophobic and hydrophilic CAs. The 90-nm nanogels (hydrodynamic diameter) exhibited a neutral surface charge and no hemolytic activity in pH 7.4 PBS, indicating stability in a simulated physiological environment and ability to retain the CAs during circulation in the bloodstream. The nanogels swelled to 120-nm in response to acidic pH and demonstrated significant membrane disruption in PBS with pH < 6.8, demonstrating the ability to rapidly release the encapsulated CAs and mediate endosomal rupture in a pH-dependent manner.
This work was supported by a grant from the National Institutes of Health (R01-EB-000246-22); The authors would like to acknowledge the assistance of Balark Chethan, Rishabh Shah, and Alina Schroeder
References:
[1] Kolishetti, N., et al. (2010). "Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy." Proceedings of the National Academy of Sciences 107(42): 17939-17944.
[2] Jabr-Milane, L. S., et al. (2008). "Multi-Functional Nanocarriers to Overcome Tumor Drug Resistance." Cancer Treat Rev 34(7): 592-602.
[3] Fisher, O. Z., et al. (2009). "Enhanced core hydrophobicity, functionalization and cell penetration of polybasic nanomatrices." Pharm Res 26(1): 51-60.