Introduction: Stem cells respond to various cues from their microenvironment (e.g., stiffness, degradation) towards numerous processes (e.g., differentiation)[1]. For example, recent work demonstrated that human mesenchymal stem cells (MSCs) encapsulated in covalently-crosslinked hydrogels required a protease-degradable microenvironment to generate traction forces and undergo osteogenesis[2]. Recently, Yes-associated protein (YAP) signaling has been implicated as a mechanical rheostat that plays an integral role in how cells interpret their mechanical environment[3], yet little work has been done to understand this signaling within 3D biomaterials. Here, we used controlled hydrogel degradation to independently tune mechanics and MSC spreading to investigate YAP signaling in 3D.
Materials and Methods: MSCs (Lonza, passage 3) were photoencapsulated in norbornene-functionalized hyaluronic acid (NorHA) hydrogels containing RGD and crosslinked with di-thiol peptides engineered to either be non-degradable (non-deg) or protease-degradable (deg) to restrict or permit cell spreading, respectively (Fig 1a). After 7 days in culture, MSC-laden gels were fixed, permeabilized, and blocked with 1% NGS followed by primary (YAP, 1:200 4°C overnight) and secondary (AlexaFluor 488, 1:200, 2 hours) antibody incubations. To visualize individual cells and nuclei, gels were also stained with rhodamine phalloidin (1:100) and DAPI (1:500), respectively. Using a Leica SP8 confocal microscope system (20x), xy-plane cross-sections were acquired (thickness >50μm, step-size 0.69μm) and reconstructed using Volocity Image Analysis Software. Actin, YAP, and nuclei volumes were determined using intensity-based thresholding. Cell Shape Index (CSI), a measure of roundness (1 = sphere) and Nuclear YAP Ratio, the normalized ratio between nuclear and cytosolic YAP were calculated for single cells in non-deg (n = 45) and deg (n = 56) gels. * (p < 0.01) indicates statistical significance.
Results and Discussion: NorHA hydrogels with variable crosslinking densities were fabricated over a range of stiffnesses (1-20 kPa) with no statistical differences in gelation kinetics, moduli, or cell viability (>90%) between the non-deg and deg gels. When quantified, MSCs cultured in deg gels (5 kPa) were almost twice as large as those encapsulated in non-deg gels (Fig 1b) with significantly lower CSI values (0.36 ± 0.02) compared to MSCs in the non-deg gels (0.60 ± 0.02) (Fig 1c,d).
Given that the deg hydrogel system supported increased MSC spreading, we next investigated YAP-mediated mechanical signaling where activation is indicated by translocation from the cytosol to the nucleus. MSCs cultured in non-deg gels (low spreading) had nearly equal amounts of nuclear and cytosolic YAP (1.04 ± 0.08 YAP ratio), whereas MSCs in the deg gels (high spreading) had about twice as much nuclear YAP (2.05 ± 0.08 YAP ratio) (Fig 1e). YAP localization and MSC spreading as measured by CSI were correlated with two distinct populations: a) High CSI, Low Nuclear YAP for non-deg gels, and b) Low CSI, High Nuclear YAP for deg gels (Fig 1f).
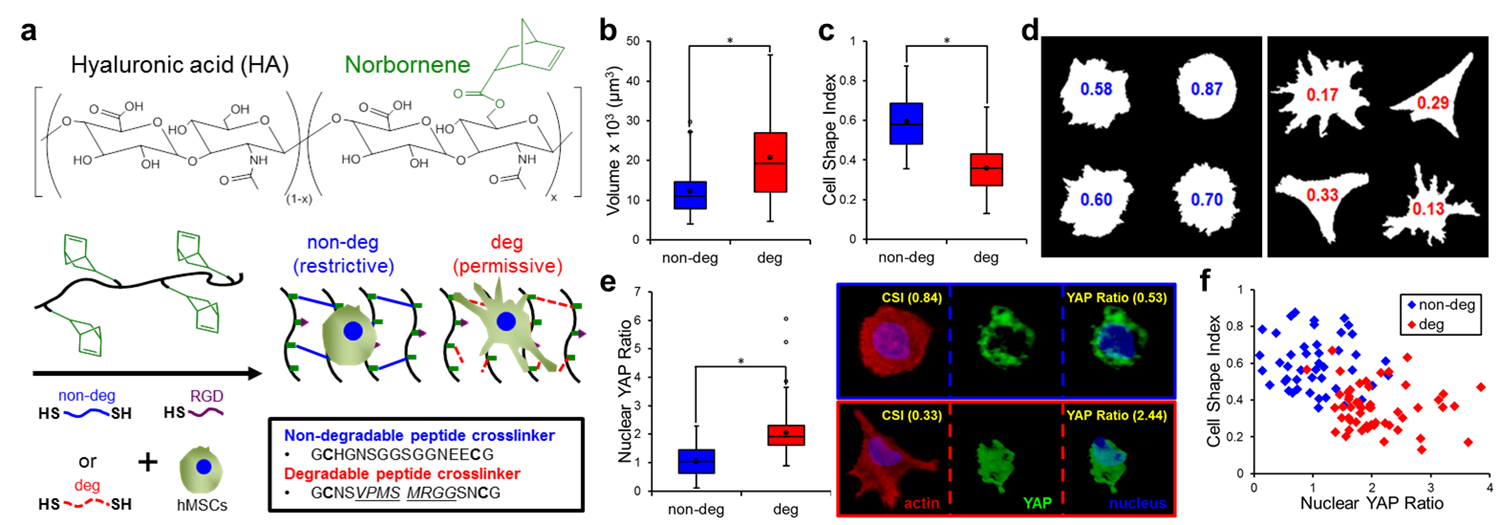
Conclusion: We developed a hydrogel system that permits independent tuning of stiffness and degradability. We applied this platform to demonstrate for the first time that hydrogel degradability in covalently-crosslinked hydrogels is necessary for MSC spreading and activation of the YAP mechanotransduction pathway in 3D.
References:
[1] Watt FM et al., Nature Reviews Molecular Cell Biology, 2013, vol. 14, 467-473
[2] Khetan S et al., Nature Materials, 2013, vol. 12, pp. 458-465
[3] Dupont S et al., Nature, 2011, vol. 474, pp. 179-183