Introduction: Therapeutic angiogenesis holds great potential within regenerative medicine approaches, where insufficient vascularization limits construct size, complexity, and anastomosis with host vasculature. Many pro-angiogenic approaches have been developed, often via delivery of angiogenic proteins or peptides. Peptides typically mimic the bioactivity of larger proteins or growth factors, and offer advantages over traditional protein delivery. However, like proteins, peptides suffer from rapid clearance and poor pharmacokinetics when delivered systemically. Therefore, a poly(ethylene glycol) (PEG) hydrogel-based platform technology was developed to control and sustain peptide drug release via matrix metalloproteinase (MMP) activity.
Materials and Methods: Nine pro-angiogenic peptides, identified from literature, were synthesized with MMP substrate modifications using solid phase peptide synthesis. In vitro screening using human umbilical vein endothelial cell (HUVEC) proliferation and tube formation assays[4] of peptides identified three that retained native pro-angiogenic peptide bioactivity in the form released upon liberation from hydrogel networks: Qk (a Vascular Endothelial Growth Factor (VEGF) mimic), and SPARC113 and SPARC118 (derived from the Secreted Protein Acidic and Rich in Cysteine). Norbornene-functionalized PEG was synthesized and reacted with MMP-releasable pro-angiogenic peptides with cysteine-based thiol functionalities to form hydrogels via thiol-ene reactions[5]. Enzymatically-responsive peptide release was assessed, as was overall hydrogel behaviors in vivo. Specifically, one month after implantation, hydrogels were collected and vascular ingrowth assessed via hemoglobin (Hb) quantification and fluorescent dextran imaging using two photon microscopy. All animal procedures were approved by the University of Rochester’s University Committee of Animal Resources.
Results and Discussion: Incorporation Qk, SPARC113, and SPARC118 into hydrogels flanked (Figure 1A) or tethered (Figure 1B) by MMP-degradable substrates successfully produced hydrogels with enzymatically-responsive peptide release behaviors. Qk, SPARC113, and SPARC118-releasing hydrogels were confirmed to release bioactive components in vitro after MMP-mediated degradation (Figure 1A). Further investigation revealed key peptide drug properties, specifically size and hydrophobicity, control the rate of hydrogel degradation and peptide release[3]. When implanted subcutaneously, SPARC113 and SPARC118-releasing hydrogels both significantly increased vascular ingrowth compared to controls without significantly affecting vessel size[1]. As the longitudinal availability of VEGF has been shown critical for bioactivity, alternate hydrogels were developed to provide temporal control over enzymatically-responsive release of Qk, the VEGF peptide mimic[2]. MMP-degradable linkers with a variety of cleavage kinetics were used to tether Qk to hydrogels formed with non-degradable peptide crosslinks (Figure 1B) [2]. Hydrogel degradation was successfully uncoupled from peptide release, and three of the linkers provided temporal control over enzymatically-responsive peptide release kinetics in vitro and in vivo[2]. Modifying the MMP-degradable linker used to tether Qk to hydrogels provided temporal control over enzymatically-responsive peptide release in vitro and in vivo. Qk was confirmed to be bioactive as released and induced significant vascularization in vivo 1 month after implantation (Figure 2)[2].
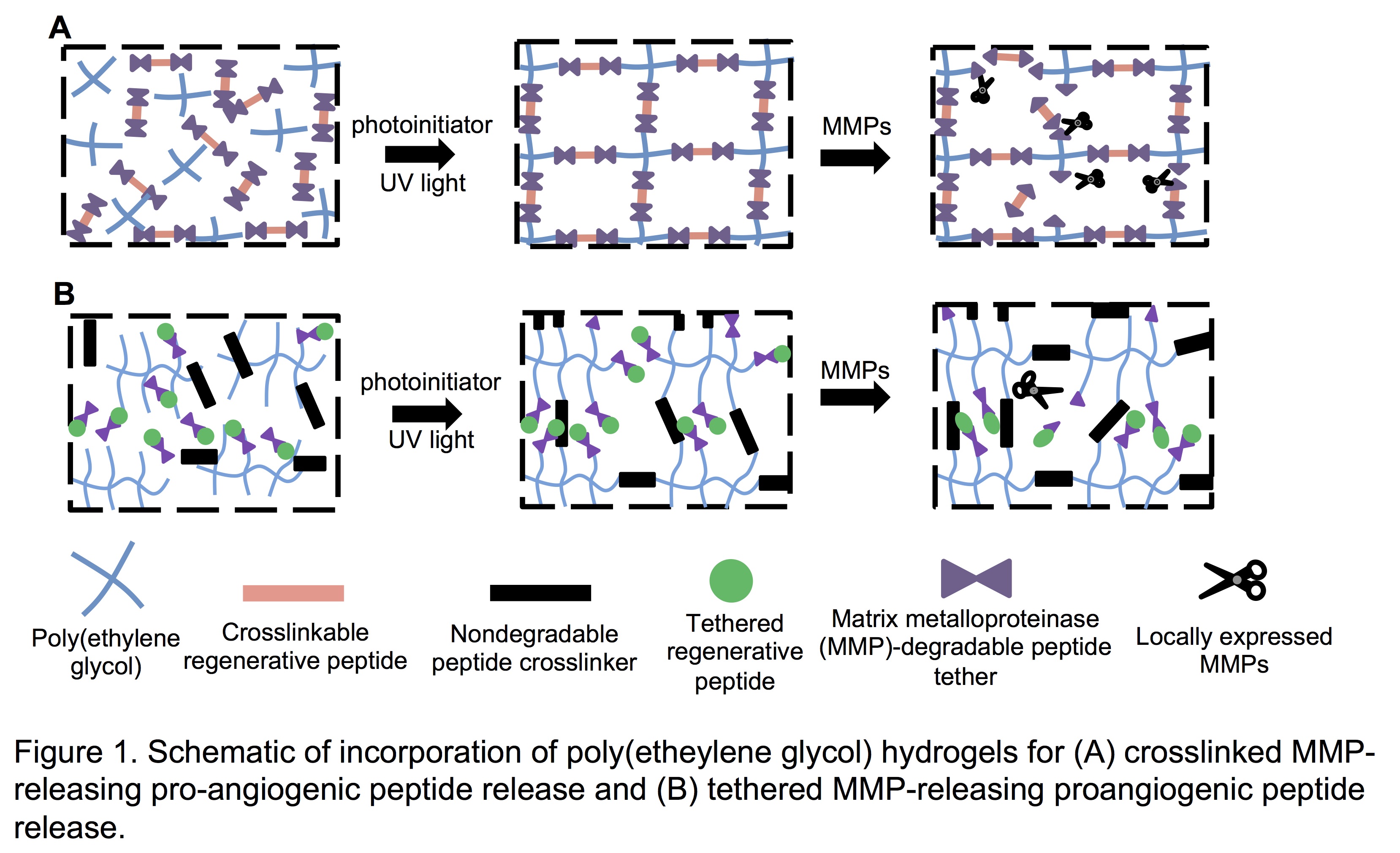
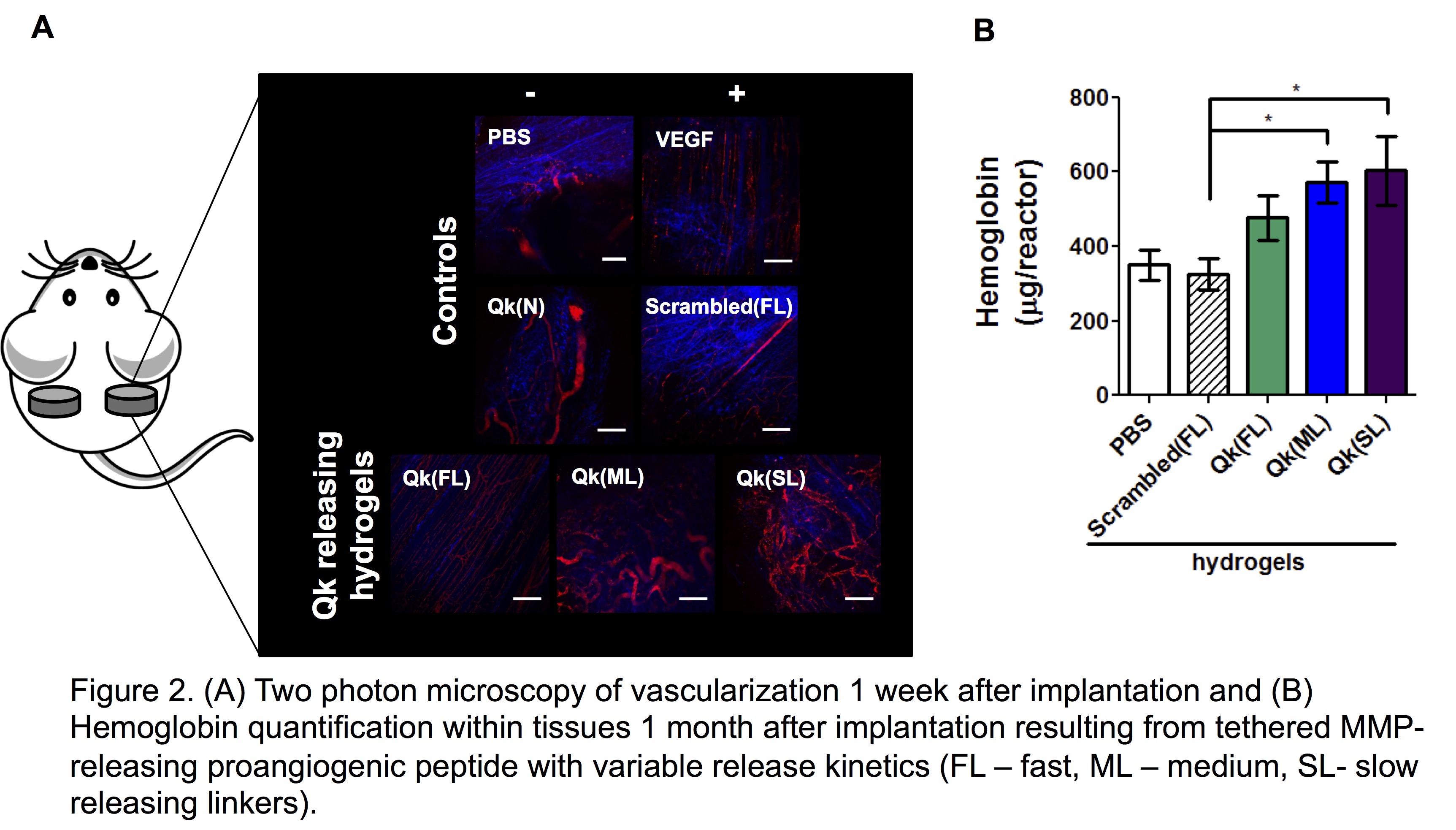
Conclusions: The hydrogels developed are a promising and versatile platform technology for stimuli-responsive delivery of therapeutic peptides from poly(ethylene glycol) hydrogels that could be adapted to release virtually any peptide or small molecule drug for myriad applications.
NIH-R01AR064200, Howard Hughes Med-into-Grad Program
References:
[1] A.H. Van Hove, K. Burke, E. Antonienko, E. Brown III, and D.S.W. Benoit. Enzymatically-responsive pro-angiogenic peptide-releasing poly(ethylene glycol) hydrogels promote vascularization in vivo. Journal of Controlled Release. In press.
[2] A.H. Van Hove, E. Antonienko, K. Burke, E. Brown III, and D.S.W. Benoit. Temporally Tunable, Enzymatically-responsive Delivery of Pro-angiogenic Peptides from Poly(ethylene glycol) Hydrogels. Advanced Healthcare Materials. In press.
[3] A.H. Van Hove, M.J. Beltejar, and D.S.W. Benoit. Development and In Vitro Assessment of Enzymatically-Responsive Poly(ethylene glycol) Hydrogels for the Delivery of Therapeutic Peptides. Biomaterials. 35 (2014) 9719-9730.
[4] R. Auerbach, R. Lewis, B. Shinners, L. Kubai and N. Akhtar. Angiogenesis assays: A critical overview. Clinical Chemistry 49, 32-40 (2003).
[5] B.D. Fairbanks, M.P. Schwartz, A.E. Halevi, C.R. Nuttelman, C.N. Bowman, K.S. Anseth. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Advanced Materials 21, 5005-5010 (2009).