Introduction: 3D bioprinting is a promising approach for tissue engineering constructs (TECs) via positioning biomaterials, growth factors, and cells with controlled spatial distribution due to its layer-by-layer manufacturing nature[1]-[5]. Hybrid TECs composed of relatively rigid porous scaffolds for structural and mechanical integrity and soft hydrogels for cell- and growth factor-loading have a tremendous potential to tissue regeneration under mechanical loading. However, despite excessive progress in the field, the current 3D bioprinting techniques and systems fall short in integration of such soft and rigid multifunctional components[6],[7]. Here we present a novel 3D hybrid bioprinting technology (Hybprinter) and its capability enabling integration of soft and rigid components for TECs.
Materials and Methods: A built-in-house novel fabrication system, called Hybprinter, employs digital light processing-based stereolithography (DLP-SLA) and molten material extrusion (MME) techniques for soft and rigid materials, respectively. In this study, poly-ethylene glycol diacrylate (PEGDA) (Mn=1,000 g/mol, Polyscience, Inc.) and poly-(ε-caprolactone) (PCL) (1.145 g/cm3, Mn = 80,000 g/mol, Aldrich Chemical Company) were used as a model material for soft hydrogel and rigid scaffold, respectively. Human umbilical vein endothelial cells (HUVECs) expressing green fluorescent protein (GFP) were used for cell incorporation in PEGDA and collagen. We evaluated the effects of process time and combinatory process on cell viability via live-dead assay.
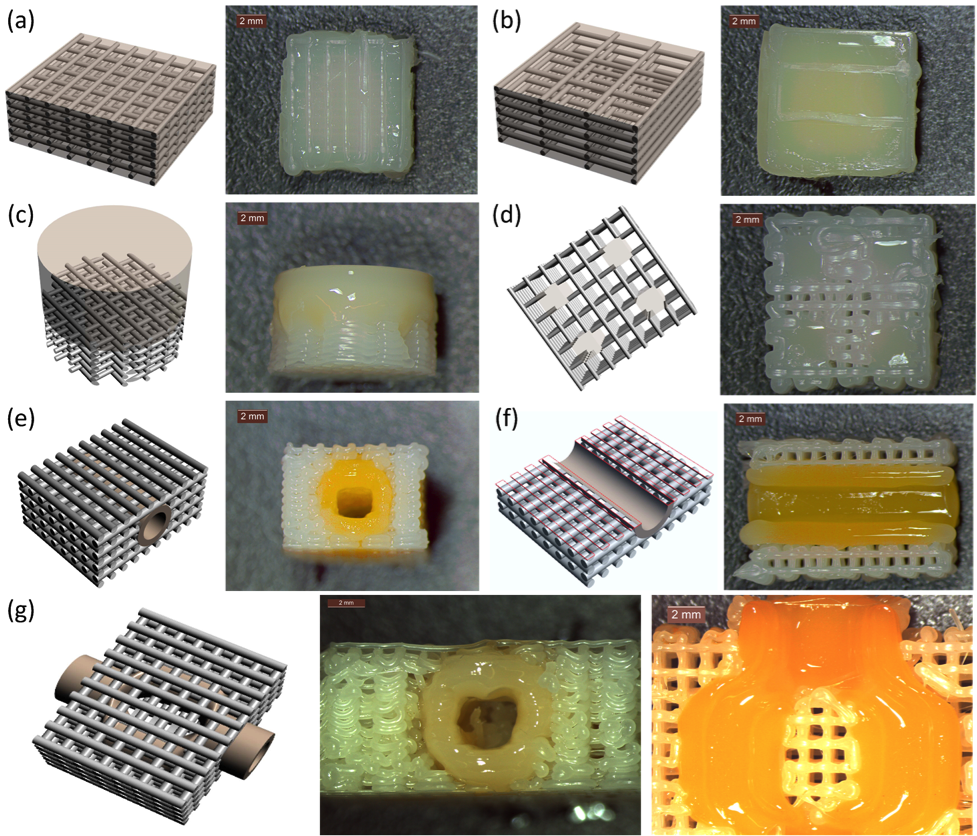
Results and Discussion: Our study has shown that geometrical accuracy, swelling ratio and mechanical properties of the hydrogel component can be tailored by DLP-SLA module. We also have demonstrated the printability of variety of complex hybrid construct designs using Hybprinter technology as shown in Figure 1. Figure 1(a) shows a 70% porous scaffold with PEGDA hydrogel filling the pores of scaffold. Such scaffold-hydrogel combination can be used for enhanced uniform 3D distribution of cells across scaffold. Figure 1(b) shows bulk hydrogel reinforced with PCL lattices of a 90% porous scaffold. The lattice struts embedded in the gel during layer-by-layer fabrication significantly improved mechanical properties of bulk hydrogel. Also, a biphasic construct composed of bottom rigid porous PCL scaffold segment and top soft PEGDA hydrogel component is shown in Figure 1(c). In addition, a representative sample composed of four hydrogel blocks within scaffold was formed as shown in Figure 1(d). Figure 1(e) demonstrates a construct composed of a straight hydrogel conduit integrated within a porous scaffold. Such construct can be utilized as connectable vascularized TECs. The cross-section of the conduit in the scaffold is presented in Figure 1(f). Also, a bifurcated conduit embedded in the porous scaffold is shown from side view and cross section in Figure 3(g). We also characterized the mechanical properties and functionality of such constructs. The compressive mechanical stiffness of a hybrid construct (90% hydrogel) was significantly higher than hydrogel itself (~6 MPa vs. 100 kPa). In addition, viability of cells incorporated within the bioprinted hybrid constructs was determined approximately 90%. Furthermore, a functionality of a hybrid construct composed of porous scaffold with an embedded hydrogel conduit was characterized for vascularized tissue engineering applications. High material diffusion and high cell viability in about 2.5 mm distance surrounding the conduit indicated that culture media effectively diffused through the conduit and fed the cells.
Conclusion: The results suggest that the developed technology is potent to form functional TECs composed of rigid and soft biomaterials.
We would like to acknowledge the financial support of the following agencies: NIH R01AR057837 (NIAMS), NIH R01DE021468 (NIDCR), DOD W911NF-14-1-0545 (DURIP), DOD W81XWH-10-1-0966 (PRORP), and Stanford Coulter Translational Seed Grant.
References:
[1] Fedorovich N E, Schuurman W, Wijnberg H M, Prins H J, van Weeren P R, Malda J, Alblas J and Dhert W J 2012 Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds Tissue Eng Part C Methods 18 33-44
[2] Mironov V, Visconti R P, Kasyanov V, Forgacs G, Drake C J and Markwald R R 2009 Organ printing: Tissue spheroids as building blocks Biomaterials 30 2164-74
[3] Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S and Sun W 2014 Three-dimensional printing of Hela cells for cervical tumor model in vitro Biofabrication 6 035001
[4] Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, Hubbard B, Colbert S and Gabor F 2012 Toward engineering functional organ modules by additive manufacturing Biofabrication 4 022001
[5] Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G and Forgacs G 2010 Tissue engineering by self-assembly and bio-printing of living cells Biofabrication 2 022001
[6] Murphy S V and Atala A 2014 3D bioprinting of tissues and organs Nat Biotechnol 32 773-85
[7] Bajaj P, Schweller R M, Khademhosseini A, West J L and Bashir R 2014 3D biofabrication strategies for tissue engineering and regenerative medicine Annu Rev Biomed Eng 16 247-76