Introduction: 3D printing of hydrogels is an additive fabrication approach that has the potential to create complex construct architectures not possible using other tissue engineering methods. While there have been a number of advances, the 3D printing of soft hydrogels such as alginate, collagen and fibrin remains challenging because these materials typically have an elastic modulus <100 kPa and deform during the layer-by-layer printing process in air. To address this we have developed a Bingham plastic support bath into which these hydrogels can be printed with high fidelity and subsequently released, using a process we term freeform reversible embedding of suspended hydrogels (FRESH). Using FRESH we demonstrate (i) a biocompatible support material that prevents print deformation during and after gelation, (ii) non-destructive removal of the support material after printing, and (iii) maintaining sterile conditions and temperature, pH, and osmolality within the physiologic range.
Methods: The Bingham plastic support bath was produced by blending a gelatin block until particle size was reduced to 55 ± 2 μm, forming a microparticulate slurry. A MakerBot Replicator 3D printer with custom syringe-pump extruder was used to print alginate, collagen type I and fibrin within the support bath in various 3D geometries based on confocal, MRI and CT imaging data. Melting the gelatin support at 37ºC released printed structures. Printed constructs were then imaged using optical microscopy and compared to the original 3D computer models to assess print fidelity.
Results: 3D printed hydrogel filaments within the support bath had a diameter of ~200 µm, demonstrating a resolution that matched the capabilities of the open-source hardware used for the 3D printer. FRESH printing the letters “CMU” in alginate with black dye added for visualization demonstrated the printing and thermally-triggered release process (fig 1A). MRI data of part of the right coronary arterial tree (fig 1B) was 3D printed using fluorescent alginate (fig 1C) with a well-defined lumen that was patent and perfusable (fig 1D). Next, we took an embryonic chick heart (fig 1E), stained the cells and extracellular matrix and imaged in 3D with multiphoton microscopy (fig 1F), generated a 3D model with internal trabeculations from the imaging data (fig 1G) and 3D printed the embryonic heart using alginate (fig 1H) with <10% dimensional variability.
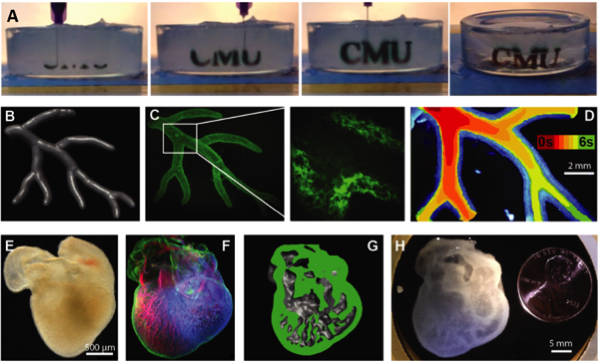
Figure 1. (A) Time lapse of the letters “CMU” FRESH printed in alginate (black dye added for visualization) and then released by melting the gelatin slurry at 37ºC. (B) A right coronary artery tree 3D model from MRI data was (C) FRESH printed, and (D) perfused. (E) An embryonic chick heart was (F) fluorescently labeled and imaged in 3D to create (G) a 3D model with internal trabeculations that was (H) FRESH printed in alginate.
Conclusions: We developed the FRESH 3D bioprinting process to create complex structures from soft, biological hydrogels. Example prints of arteries and hearts and dimensional analysis compared to original 3D models validates the fabrication of complex 3D structures with high fidelity while using inexpensive open-source technologies. Future work is focused on cellularizing these constructs to create functional tissues.
National Institutes of Health (DP2HL117750); National Science Foundation (CAREER Award 1454248)