Cardio-vascular diseases are the main cause of death emphasizing the need to identify alternatives in patient treatment. The application of shear-sensitive liposomes loaded with a vasodilator for targeted drug delivery belongs to these alternative therapies[3]. The hemodynamic shear-stress differences between healthy and constricted vessels can be used as a purely physical trigger for drug release[1],[2]. Although liposomes are established delivery containers they are usually recognized as foreign bodies by the immune system and therefore cause non-desired side effects.
Figure 1 schematically summarizes the study. Complement activation, as the essential factor of the recognition, was tested in human sera collected from six blood donors and, in a preliminary study, with the established and sensitive Yorkshire pig model. We have tested the complement cascade activation by empty and nitroglycerin-loaded liposomes formulated from the artificial phospholipid 1,3-diamidophospholipid 1,3-palmitoylamido-1,3-deoxy-sn-glycero-2-phosphatidyl-choline (Pad-PC-Pad) in vitro and compared the results with saline and zymosan, see Fig. 1. Furthermore, we have evaluated liposomal formulations with respect to zymosan in vivo and included Pad-PC-Pad with PEGylated lipids to prevent the aggregation of the nano-containers, i.e. of the lentil-shaped liposomes about 100 nm in diameter. During these animal experiments with nine pigs the hemodynamics, i.e. the heart rate, the pulmonary arterial blood pressure, and the systemic arterial pressure, were recorded.
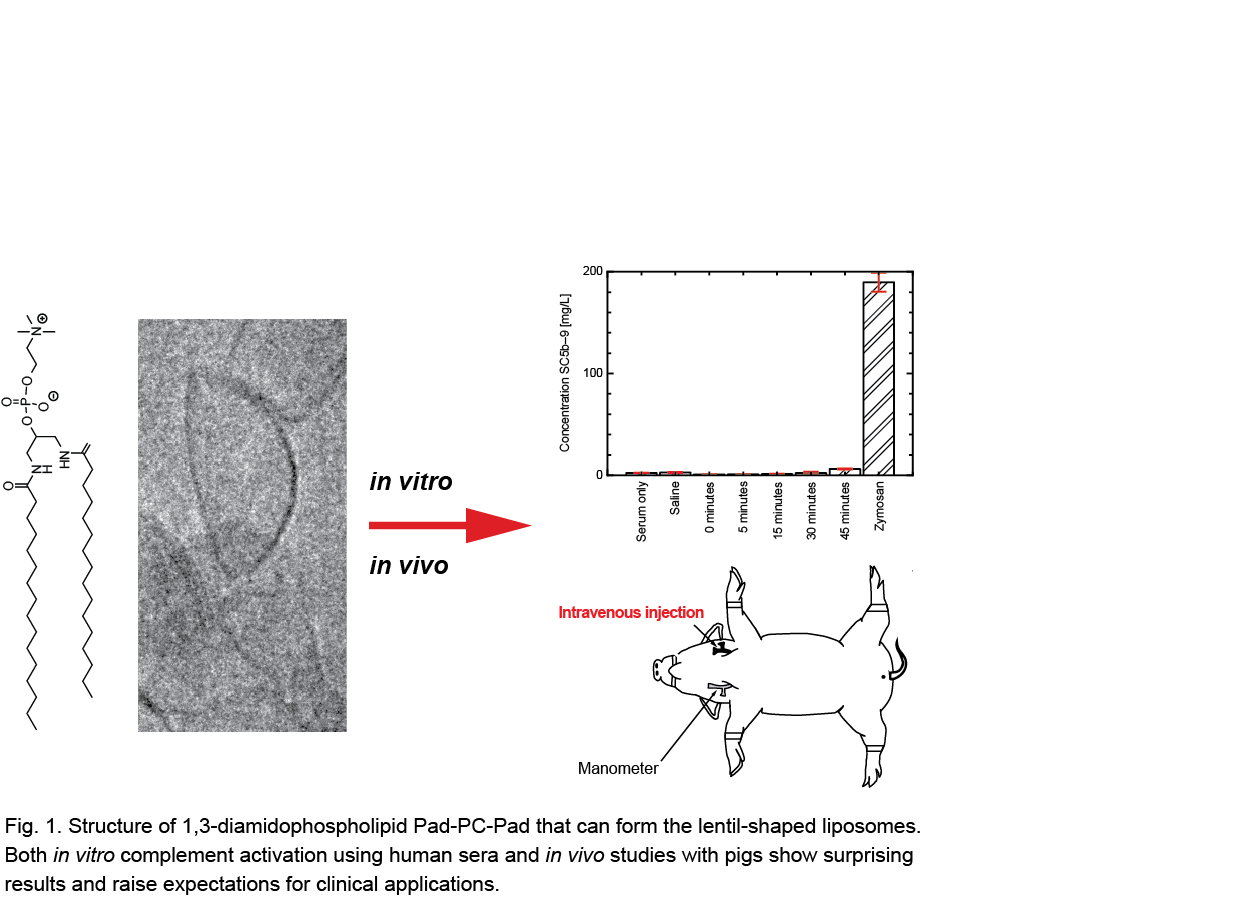
The results of the in vitro experiments show complement activation, which is very low compared to the FDA-approved liposomal formulations from the natural 1,2-diesterphospholipid DPPC. In the in vivo studies with pigs, anaphylactic reactions and other significant haemodynamic changes were hardly observed or even absent at liposome doses much higher than the ones used in experiments with the FDA-approved formulations Doxil® and AmBisome®.
The encouraging results obtained in the present pilot study demonstrate that Pad-PC-Pad liposomes do not show the characteristic anaphylactoid reactions and thus took a first-line barrier toward clinical application. In this sense, the results of the present communication will initiate research and development of this special class of mechano-sensitive nano-containers for targeted drug delivery by a purely physical trigger.
References:
[1] M.N. Holme, G. Schulz, H. Deyhle, T. Weitkamp, F. Beckmann, J.A. Lobrinus, F. Rikhtegar, V. Kurtcuoglu, I. Zanette, T. Saxer, B. Müller: Complementary X-ray tomography techniques for histology-validated 3D imaging of soft and hard human tissues using plaque-containing blood vessels as examples, Nature Protocols 9 (2014) 1401–1415
[2] M. N. Holme, G. Schulz, H. Deyhle, S. E. Hieber, T. Weitkamp, F. Beckmann, J. Herzen, J. A. Lobrinus, F. Montecucco, F. Mach, A. Zumbuehl, T. Saxer, B. Müller: Morphology of atherosclerotic coronary arteries, Proceedings of SPIE 8506 (2012) 850609
[3] M. N. Holme, I. A. Fedotenko, D. Abegg, J. Althaus, L. Babel, F. Favarger, R. Reiter, R. Tanasescu, P-L. Zaffalon, A. Ziegler, B. Müller, T. Saxer, A. Zumbuehl: Shear-stress sensitive lenticular vesicles for targeted drug delivery, Nature Nanotechnology 7 (2012) 536-543