Introduction: Particulated cartilage autograft and fresh osteochondral allograft has been used clinically to treat cartilage defects[1]. However, both these current cartilage repair techniques present drawbacks. Autograft cartilage can cause donor site morbidity and there is a limit to the amount than can be harvested in order to fill a large defect. Fresh osteochondral allograft with fresh living cells has a short shelf-life making it difficult to manage inventory and resulting in tissue being discarded due to limited shelf-life. We propose cryopreservation of allograft cartilage to extend the shelf-life but retain viable and active chondrocytes within particulated cartilage fragments. In this study, we evaluated the viability and bioactivity of cryopreserved human cartilage fragments compared to fresh tissue.
Materials and Methods: Human cartilage fragments were harvested from seven adult human condyles (LifeLink Tissue Bank) and sieved between 1-0.1mm. The fragments were then cryopreserved for 12, and 24 weeks. Fresh unfrozen fragments served as a control. A subset of the cartilage fragments at each time point was digested overnight with collagenase to dissociate the tissue and isolate chondrocytes. The viability of the chondrocytes was determined with nucleic acid binding dyes acridine orange (AO) and propidium iodide (PI). Live nucleated cells fluoresce green and the dead nucleated cells fluoresce red. The cellular viability was determined using the Cellometer K2 (Nexcelom). The remaining cartilage fragments were placed in 24-well transwell plates. All fragments were cultured at 37°C, 5% CO2 in complete media consisting of DMEM+GlutaMax (Gibco) supplemented with 10% FBS, 1X antibiotics, and 1X antimycotic for 8 weeks. After 8 weeks the constructs were encased in HistoGel (Thermo Fisher) and collected from the transwells. The constructs were fixed in 10% formalin and embedded in paraffin wax and 5μm sections were prepared. Sections were stained with hematoxylin and eosin (H&E).
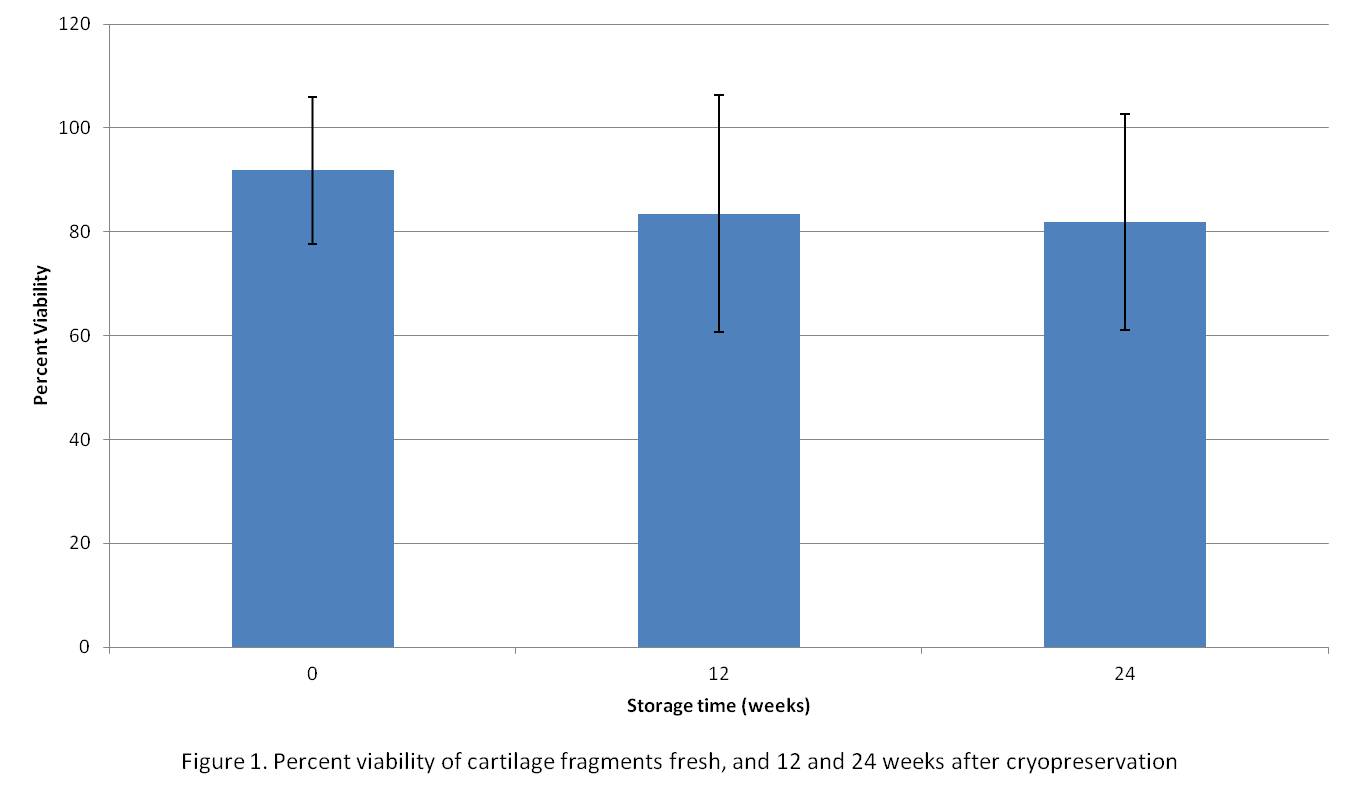
Results and Discussion: The viability of fresh cartilage was about 92%. The viability of the cryopreserved chondrocytes at 12 and 24 weeks was 84% and 82% respectively and was not significantly different compared to fresh tissue (Figure 1).
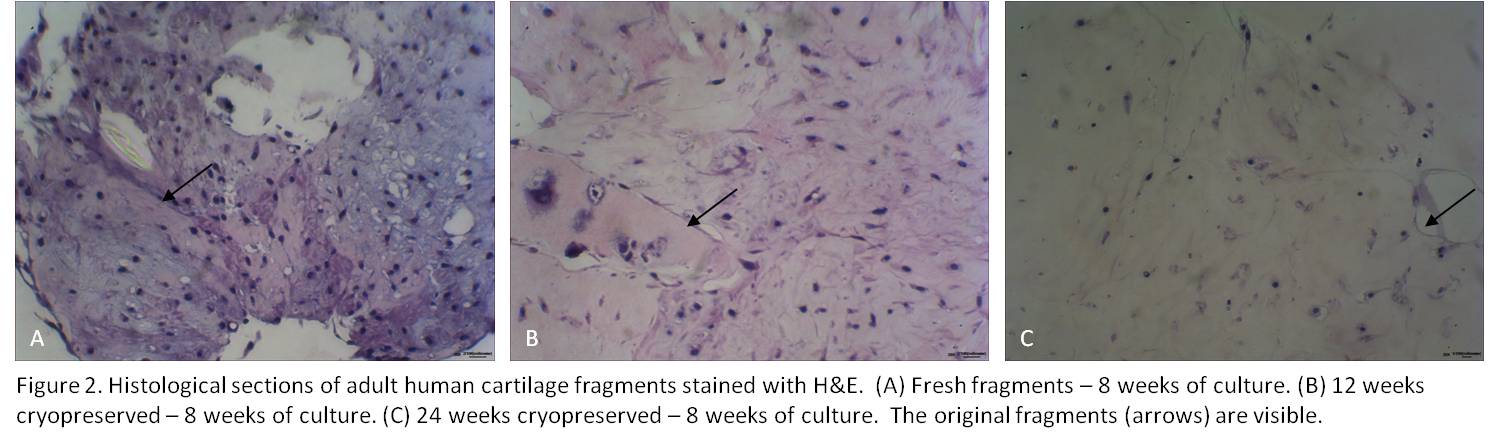
Fresh cartilage fragments displayed hypercellular regions indicative of chondrocytes outgrowth and fragment fusion after 8 weeks of culture (Figure 2A). The cryopreserved fragments following 12 and 24 weeks of cryopreservation displayed cellular outgrowth and fragment fusion following 8 weeks of culture as well (Figure 2B-C).
Conclusion: Several studies have shown that cartilage is a source of chondrocytes which can be used to treat cartilage defects. We investigated a processing technique for adult allograft cartilage which entails particulation and cryopreservation. In this study the cryopreserved fragments maintained high viability and both fresh and cryopreserved human cartilage fragments displayed robust outgrowth. Cryopreservation of adult cartilage with a prolonged shelf-life may provide an attractive alternative for treating cartilage defects. Future studies should evaluate the bioactivity of these fragments in-vivo.
References:
[1] J. Farr, B.J. Cole, S. Sherman, V. Karas, "Particulated articular cartilage: CAIS and DeNovo NT," The Journal of Knee Surgery. Vol. 25, Mar. 2012.