Introduction: Age related macular degeneration (AMD) is the leading cause of irreversible vision loss in the developed world[1],[2] and manifests as either dry or wet AMD[1],[2]. In dry AMD, the retinal pigmented epithelium cells (RPEs) and Bruch’s membrane (BM) are damaged, leading to photoreceptor dysfunction and death, and subsequent vision reduction and/or complete loss[2],[3].
Aim: The aim of this project was to develop a biomimetic scaffold for the treatment of dry AMD. Here, we focused on developing electrospun polymeric scaffolds, namely: poly(ε-caprolactone) (PCL), poly(L-lactide-co-glycolide) (PLGA), poly(D,L-lactide-co-L-lactide) (PDLLA) and poly(L-lactide) (PLLA) that mimicked the microenvironment of native BM. Additionally, we investigated the in vitro efficacy of these scaffolds to support the proliferation and functionality of human fetal RPE cells (hfRPEs).
Materials and Methods: Electrospun membranes were fabricated from the above-mentioned polymers via electrospinning, as described previously[2],[4]. SEM was used to determine fibre diameter, scaffold thickness, and porosity of electrospun membranes. Surface roughness and surface stiffness were measured using AFM at 1 µm/s and a 5 nN load. In in vitro studies, hfRPEs were seeded (10,000/cm2) on electrospun membranes, which were then assessed at various time points via transepithelial electrical resistance (TER), SEM, q-PCR, and immunohistochemistry.
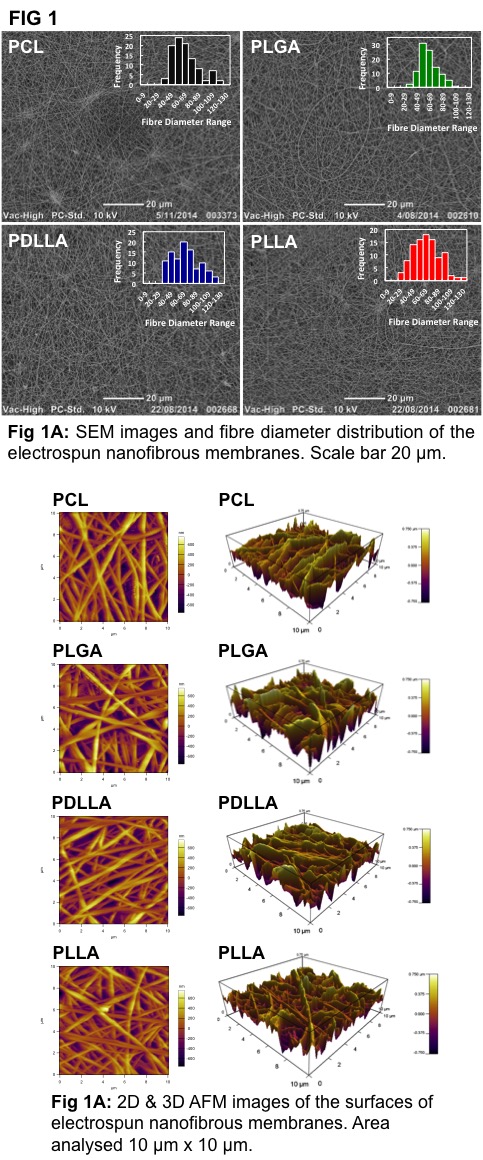
Results and Discussion: Electrospun polymeric scaffolds with average fibre diameters of 70 nm, the first of its kind, and thicknesses < 1 μm, coupled with porosities > 40%, were fabricated via electrospinning, thereby mimicking the inner collagenous layer of native BM (thickness 1.4 μm and collagen fibre diameter 60-70 nm)[5] (Fig 1).
These electrospun scaffolds supported in vitro attachment and monolayer formation of hfRPEs over 8 weeks, evidenced by an increase in TER (Fig 2A) and SEM images (Fig 2B).

Importantly, these scaffolds supported and maintained hfRPE functionality (phagocytosis) and phenotype (immunostaining and q-PCR) (Fig 3). In fabricating membranes with fibre diameters < 70 nm (Fig 1A), we were able to create scaffolds with high surface roughnesses (RMS > 250 nm) (Fig 1B). This high surface roughness, coupled with varying surface stiffnesses (PCL: 16 N/m, PLGA: 24 N/m, PDLLA: 31 N/m, PLLA: 48 N/m) caused the hfRPEs seeded on these scaffolds to behave differently. In particular, hfRPEs seeded on PLLA based electrospun membranes exhibited the highest TER values (Fig 2A), characterized with early signs (day 2) of microvilli formation (Fig 2B). Additionally, hfRPEs seeded on PLLA based electrospun membranes expressed mature RPE markers, validated by q-PCR and immunostaining (Fig 3), thereby creating native-like tissue in vitro. This formation of native-like tissue in vitro could be due to the combined effect of mimicking the native BM’s microenvironment and high surface stiffness.

Conclusion: Electrospun scaffolds that mimicked the microenvironment of native BMs and supported the proliferation and maturation of hfRPEs were successfully fabricated via electrospinning, especially PLLA based membranes. Electrospun PLLA based membranes are therefore appropriate for exploration as a scaffold for the transplantation of RPE cells, aimed at treating dry AMD. Our plan for the future is to fabricate cGMP compliant biomimetic PLLA based membranes, and carry out pre-clinical animal studies.
Clem Jones Foundation in Brisbane, Queensland, Australia
References:
[1] Fernández-Robredo, P.; Sancho, A.; Johnen, S.; Recalde, S.; Gama, N.; Thumann, G.; Groll, J.; García-Layana, A. Journal of ophthalmology. 2014, pp 1–13.
[2] Liu, Z.; Yu, N.; Holz, F. G.; Yang, F.; Stanzel, B. V. Biomaterials. March 2014, pp 2837–2850.
[3] Jha, B. S.; Bharti, K. Current stem cell reports. June 2015, pp 79–91.
[4] Surrao, D. C.; Hayami, J. W. S.; Waldman, S. D.; Amsden, B. G. Biomacromolecules. December 12, 2010, pp 3624–3629.
[5] Booij, J. C.; Baas, D. C.; Beisekeeva, J.; Gorgels, T. G. M. F.; Bergen, A. A. B. Progress in retinal and eye research. December 31, 2009, pp 1–18.