Introduction: Resorbable phosphate based glasses (PBG) can be tailored to deliver ions over different dissolution periods and this can be controlled further and applied via thin film coatings for orthopaedic implants[1],[2]. PBG compositions such as the P2O5-40 MgO-24 CaO-16 Na2O-16 Fe2O3-4 mol%[3] are cytocompatible whilst others such as P2O5-30 CaO-60 Na2O-7 TiO2-3 mol% have exhibited bioactive behaviour in vitro[4].
Experimental Methods: Quinternary PBG targets were produced by melt-quenching. These were then used as targets for RF magnetron sputtering onto Ti6Al4V discs. FIB-SEM cross sectional milling was used to observe interfacial characteristics. FTIR, XRD, EDX, XPS and NMR have been employed to make a structural comparisons between melt quenched glasses (MQG) and sputtered coatings (SC). Static contact angle with distilled water was examined. The coated samples and MQG rods were weighed and submerged in distilled water (dH2O) or phosphate buffer saline (PBS) and measured at time points up to 2 days and 28 days to determine the mass loss due to degradation.
Results and Discussion: Notably, SC and MQG phosphate based glasses of similar chemical composition were found to be structural dissimilar, attributed to thermal history and coating surface etching. Coatings of 2.67 um were found to fully degrade in dH2O, showing a t1/2 degradation profile in the first 2 hours, followed by a linear dependence from 2-24 hours until the coating was entirely degraded.
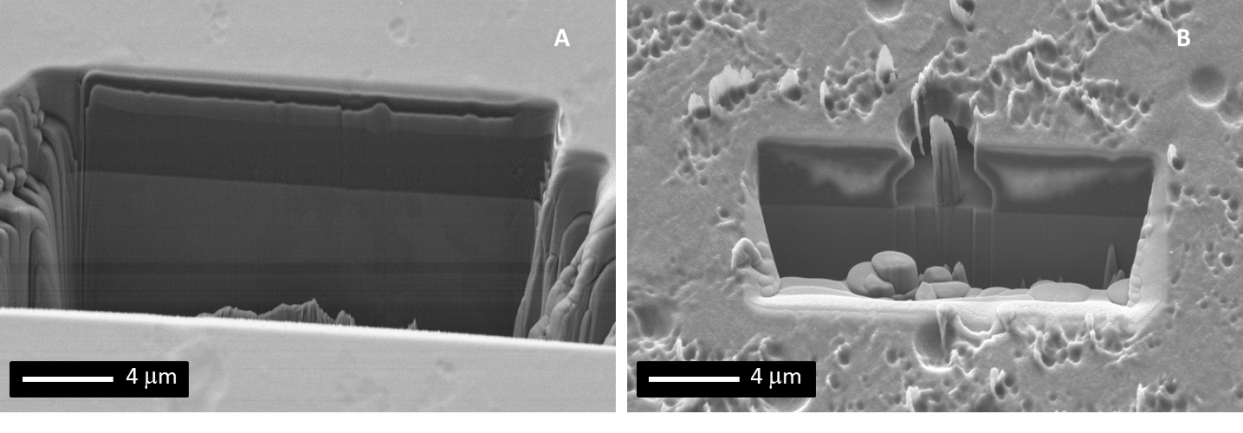
The enhanced hydration ability of the SC was confirmed by contact angle <1° with dH2O in comparison to 25° for MQG (Figure 2). XPS results suggested an increased ratio of (P-O-P) to (PO- /P=O) and mostly (PO3)- Q2 species, in comparison to the MQG counterpart which contained mostly (PO4)3- Q0 species and (P2O7)4- Q1 species, which may be responsible for the early t1/2 dissolution profile (Figure 2). Following 16 h of degradation in dH2O milling through a dissolution pit revealed that dissolution had preferentially occurred in locations and consequently extended through to the Ti6Al4V substrate prior to lateral spreading (Figure 1B). In contrast dissolution of the SC in PBS was halted by 48 hours as an X-Ray amorphous Na/Fe/Phosphate precipitation barrier had formed and showed no further signs of dissolution over a 28 d test period.
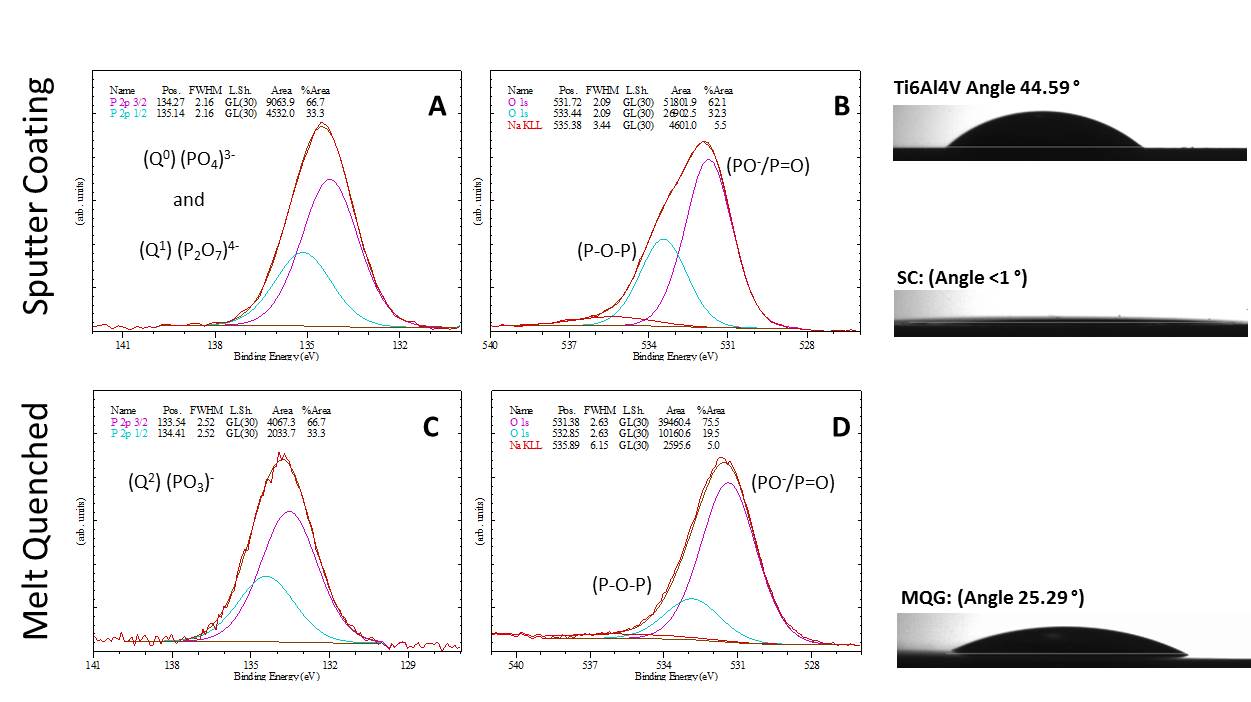
NMR comparison of a second composition indicated variation in Q structure such that SC glasses were composed of 9.4% Q0, 45.2% Q1 and 45.4% Q2, compared to 0.8, 67.3 and 31.9% respectively for MQG .
Conclusions: Sputtering of thin films (PBG) is a promising method for delivering a controlled release of therapeutic ions to an implant site. Furthermore the ability to precipitate a stable layer in physiological media has been observed in PBS. Further work has shown Hydroxyapatite precipitation in simulated body fluids and further NMR characterisation will be presented. These results could have distinct implication for assisting in the process of osseintegration.
This work was funded through MeDe Innovation, the EPSRC Centre for Innovative Manufacturing in Medical Devices, under grant number EP/K029592/1.
References:
[1] J.C. Knowles, Phosphate based glasses for biomedical applications, J. Mater. Chem., 13 (2003) 2395-2401.
[2] B. Stuart, M. Gimeno-Fabra, J. Segal, I. Ahmed, D.M. Grant, Preferential sputtering in phosphate glass systems for the processing of bioactive coatings, Thin Solid Films, DOI (2015).
[3] M.S. Hasan, I. Ahmed, A.J. Parson, G.S. Walker, C.A. Scotchford, Material characterisation and cytocompatibility assessment of quinternary phosphate glasses, J. Mater. Sci. - Mater. Med., DOI (2012).
[4] T. Kasuga, T. Fujimoto, Y. Hosoi, M. Nogami, Calcium phosphate invert glasses and glass-ceramics with apatite-forming ability, Bioceramics 15, 240-2 (2003) 265-268.