Introduction: Nanogels have been widely investigated in a wide range of biomedical applications. Temperature-responsive nanogels based on poly(N-isopropylacrylamide) (PNIPAM) have attracted particular interest due to their ability to change their diameter, hydrophobicity, pore size, and surface charge as a function of temperature; functionalized PNIPAM nanogels have been produced to enable analogous responses to pH changes. However, in the context of biological applications, PNIPAM-based nanogels are limited in that there is no way to ensure the degradability and thus clearance of the nanoparticles over time. Herein, we describe a strategy to overcome this limitation by forming nanogels by thermally-driven self-assembly of well-defined hydrazide and aldehyde-functionalized PNIPAM oligomers, which when mixed form covalent but degradable hydrazone crosslinks[3]. By maintaining the molecular weight of the oligomers below the kidney clearance limit, we can facilitate renal clearance of the synthetic polymer-based nanogel network.
Materials and Methods: Hydrazide-functionalized PNIPAM (PNIPAM-Hzd) was prepared by copolymerization of N-isopropylacrylamide (NIPAM) with acrylic acid (15 mol%) in the presence of thioglycolic acid (TGA) as a chain transfer agent to control molecular weight followed by carbodiimide-mediated coupling of a large (10x) excess of adipic acid dihydrazide. Aldehyde-functionalized PNIPAM (PNIPAM-Ald) was prepared by copolymerization of NIPAM with N-(2,2-dimethoxyethyl)methacrylamide (15 mol%) in the presence of TGA followed by acid-catalyzed hydrolysis (1M HCl) to expose the aldehyde groups. For preparing pH-responsive nanogels, precursors polymers were co-polymerized with 27mol% N,N-dimethylamino ethyl methacrylate (DMAEMA, cationic), acrylate hydrazide and acrylic acid (for hzd-polymers, anionic) or acrylic acid (for ald-functionalized polymers, anionic). All precursor polymers were characterized via titration analysis, NMR, and gel permeation chromatography. Nanogels were formed by heating solutions of PNIPAM-Hzd (0.5-2 wt% in water) above its lower critical solution temperature (LCST) to form a stable nanoaggregate that was subsequently crosslinked via addition of PNIPAM (5-20 wt% relative to PNIPAM-Hzd) (Fig. 1d).
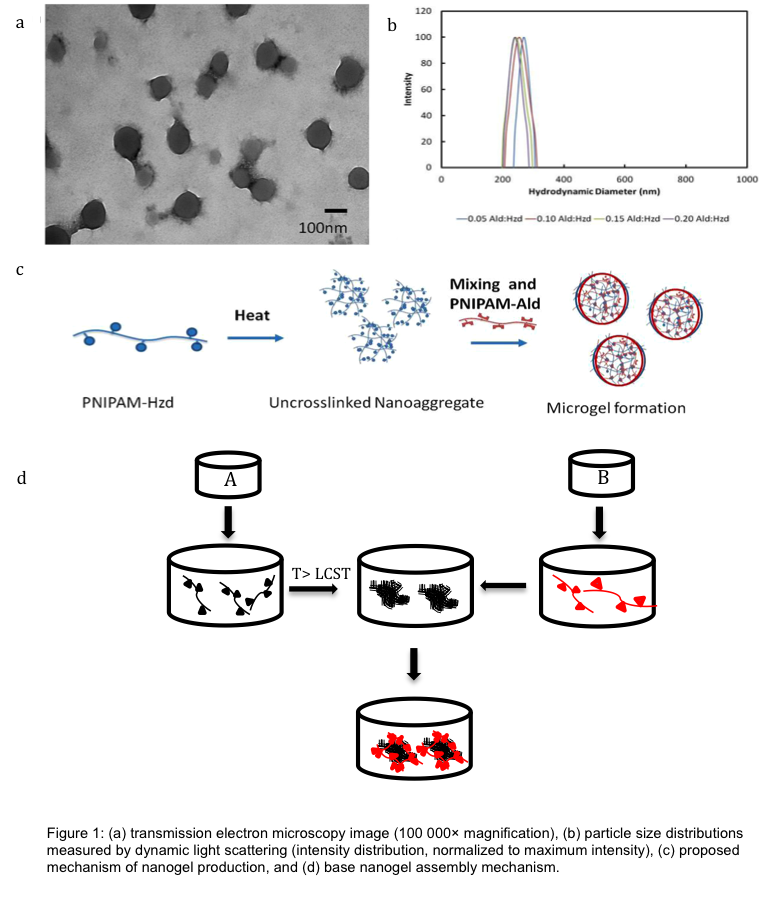
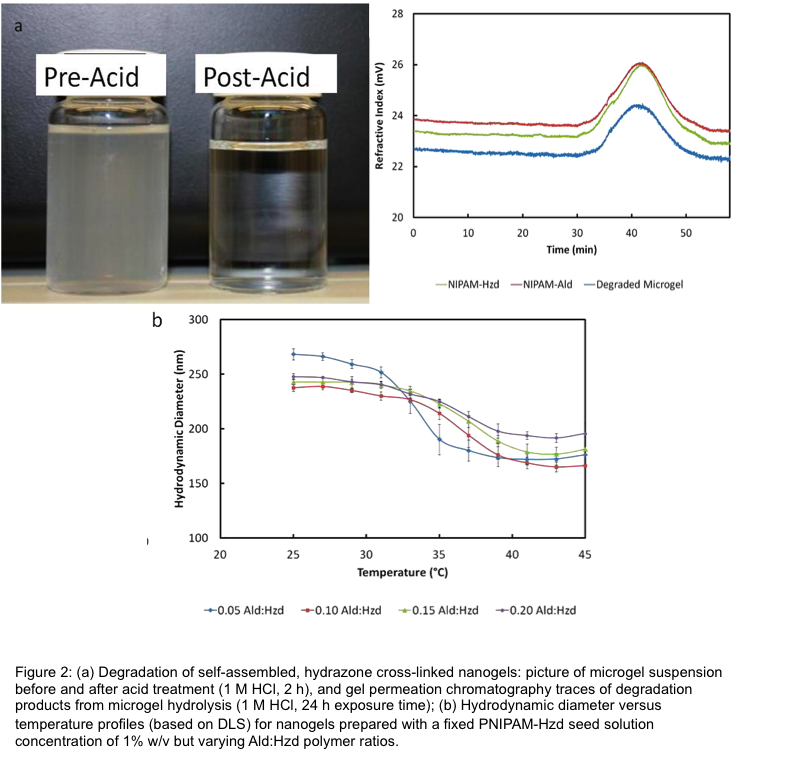
Results and Discussion: Nanogels with remarkably monodisperse size distributions (polydispersity < 0.1 via dynamic light scattering) were produced following self-assembly (Fig. 1a/b). Self-assembly at a temperature just above the LCST of the seed PNIPAM-Hzd polymer yielded the most monodisperse particles. Increasing the mixing time of the PNIPAM-Ald crosslinker, reducing the concentration of seed PNIPAM-Hzd polymer, or increasing the concentration of the crosslinking PNIPAM-Ald polymer all resulted in reduced nanogel sizes[3]. Nanogels hydrolyzed slowly (over months) back into their oligomeric precursors and maintained the discontinuous phase transition behavior typical of conventional PNIPAM nanogels (Fig. 2).
Furthermore, drug can be co-self-assembled with the polymers, leading to large (>90%) encapsulation efficiencies for moderately hydrophobic drugs e.g. dexamethasone that are significantly higher than typically achieved via conventional diffusion-based drug loading.
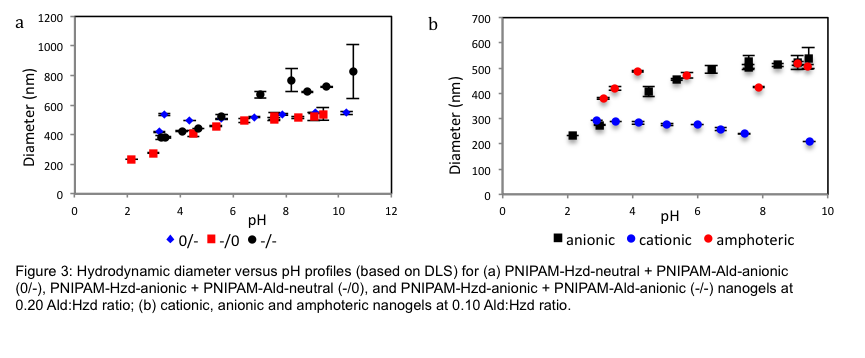
Incorporation of pH-ionizable monomers into the prepolymers facilitated the production of dual pH/temperature-responsive nanogels with tunable charge densities and pH responses[2]. Nanogels with base or acid-induced swelling, or amphoteric nanogels demonstrating dual swelling behavior, can be generated with responses consistent with conventional nanogels while preserving degradability (Fig. 3b). Different swelling responses can also be achieved depending on where the charged groups are incorporated during the self-assembly process (Fig. 3a)[1].
Conclusion: Degradable analogues of conventional thermoresponsive and/or pH responsive nanogels can be fabricated via a simple self-assembly procedure which is rapid (<10 minutes), scalable, and highly tunable to generate nanogels of different sizes and swelling responses while maintaining good monodispersity. Successful drug loading further showcases the high potential applicability of these materials in biomedical applications.
Natural Sciences and Engineering Research Council of Canada (NSERC); Dr. Hoare and his research team at McMaster University
References:
[1] Hoare, T.; McLean, D. “Kinetic Prediction of Functional Group Distributions in Microgels”. Journal of Physical Chemistry B, 2006, 110, 20327-20336.
[2] Patenaude, M.; Hoare, T. “Injectable, Mixed Natural-Synthetic Polymer Hydrogels with Modular Properties”. Biomacromolecules, 2012, 13, 369-378.
[3] Sivakumaran, D.; Mueller, E.; Hoare, T. “Temperature-Induced Assembly of Monodisperse, Covalently Cross-Linked, and Degradable Poly(N-isopropylacrylamide) Microgels Based on Oligomeric Precursors”. Langmuir, 2015, 31, 5767-5778.