Introduction: For reconstruction of critical-size bone defects a three-dimensional scaffold is beneficial to support tissue healing. This process can be enhanced with additional factors and drugs that support bone turnover. Sodium alendronate (Aln), popular antiosteoporosis drug, exhibits strong inhibitory effect against bone resorption, performed by osteoclasts[1]. Therefore a design of scaffolds assuring both mechanical support, bone tissue ingrowth and a controlled Aln release seems very viable for an effective, long-term clinical outcome. Titanium dioxide (TiO2) has been reported as a plausible candidate as it is biocompatible and it has the ability to directly bond to bone tissue[2].
The aim of this study was to: i) modify the surface of TiO2 scaffolds with poly(L-lactide-co-glycolide) (PLGA) microparticles (MPs) loaded with Aln; ii) evaluate its in vitro release profile; iii) study their performance in contact with bone forming and bone resorbing cells.
Experimental Methods: TiO2 scaffolds were manufactured by polymer sponge replication method and sintering[2],[3]. PLGA (85:15, Mn=80 kDa) MPs loaded with Aln were prepared by solid/oil/water method. Defined amounts of MPs suspended in 0.2 or 0.02% solutions of sodium alginate (Alg) were introduced on the TiO2 scaffolds by drop seeding and cross-linked with 50 mM CaCl2. Two batches of scaffolds were prepared with the use of 0.2 or 0.02% Alg and containing in total 0.5 mg of Aln. In vitro release study was performed in PBS by diffusion through semipermeable membrane (Zellu TransRoth, MWCO 12 kDa) and spectrophotometric quantitative analysis of Aln by orthophthaldialdehyde method[4]. The scaffolds were studied in contact with MG63 osteoblast-like cells and RAW 264.7 monocyte-like cells differentiated towards osteoclasts by RANKL (receptor activator of nuclear factor kappa-B ligand). Viability, morphology and proliferation of the cells were evaluated[4].
Results and Discussion: TiO2 scaffolds were highly porous (with total porosity of 90%) with oval interconnected pores of 400 µm in size, and their microstructure was very similar to that of natural cancellous bone. The average diameter of MPs was 4.1±2.1 μm with Aln encapsulation efficiency of 71% and loading efficiency 7.7%. SEM pictures (Figure 1) show high effectiveness of suspending MPs in Alg and drop seeding procedure. Differences in concentration of Alg had no impact on attachment and distribution of MPs on the scaffold pore walls. Results of in vitro release study (Figure 2) show initial burst followed by slow, sustained release of Aln for both systems. Alg concentration did not influence drug release kinetics. In vitro studies showed that the scaffolds were cytocompatible with MG-63 osteoblast-like cells and it inhibited RANKL-mediated osteoclastic differentiation of RAW 264.7 cells.
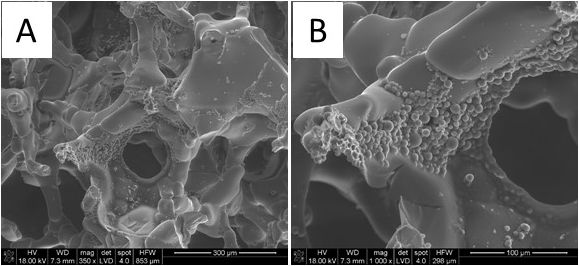
Figure 1 SEM microphotographs of TiO2 scaffold containing Aln loaded PLGA MPs immobilized with 0.02% Alg (magnification: A. 350x and B.1000x)
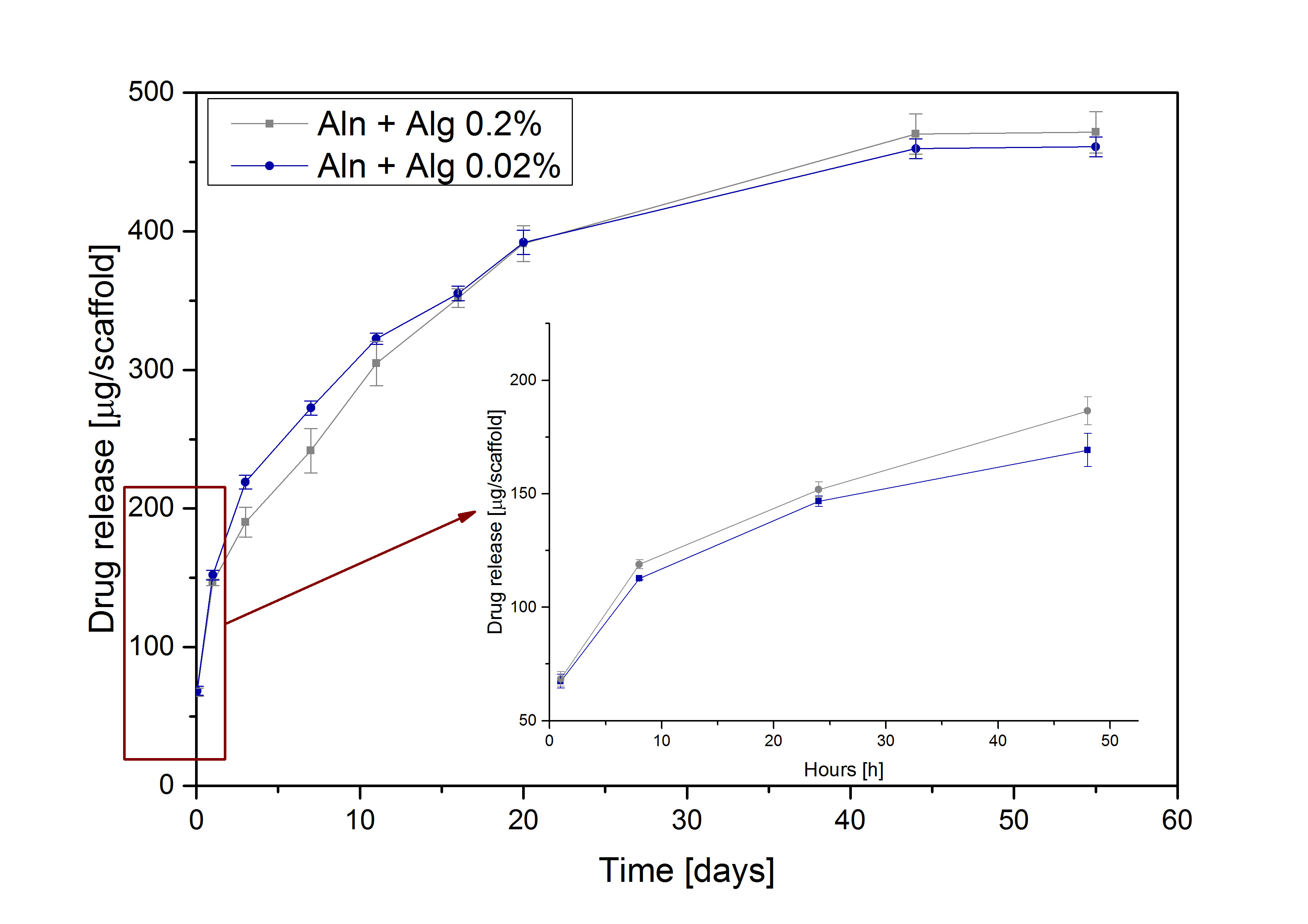
Figure 2 In vitro cumulative Aln release from TiO2 scaffolds containing MPs suspended in 0.2% and 0.02% Alg
Conclusion: The study showed that immobilization of MPs loaded with Aln on TiO2 scaffolds was successful, prolonged Aln release was achieved and the drug released from the developed system retained its biological activity.
Polish National Science Centre (Grant no: 2013/09/N/ST8/00309) and Norwegian Research Council grant no. 228415 provided financial support to this project.
References:
[1] Johnson-Lynn S. et al, Current Orthopaedics, 22: 336-340, 2008
[2] Tiainen H. et al., Acta Biomater, 8:2384–2391, 2012
[3] Rumian L. et al., Acta Bioeng Biomech, 17(1):3-9, 2015
[4] Posadowska U. et al., Int J Pharma, 485:31-40, 2015