Introduction: Following percutaneous coronary intervention Vascular Closure Devices (VCDs) are more and more often used to reduce time to ambulation, increase patient comfort, and possibly reduce complications compared with traditional manual compression. Newer techniques include complicated, more or less automated suture devices, local application of pads or using metal clips and staples. These techniques often have the disadvantage of being time consuming, expensive or not efficient enough[1]. The VCD failure rate in association with vascular complications of 2 – 9.5 %, depending on the type of VCD, is still not acceptable[2].
Aim of this study is to develop a self-expanding vascular closure device made from a bioresorbable elastic polymer that can be applied through the placed introducer sheath. The advantages of the new QVCD are cost efficient production by injection molding, quick application, easy instrumentation and matched degradation time for early ambulation of the patients[3].
Materials and Methods: Bioresorbable block-co-polymers with soft-segments of glycolide, ε-caprolactone and trimethylene carbonate have been synthesized. The chemical and mechanical degradation were determined in in vitro tests with injection molded plates in Sörensen’s buffer solution. The best fitting polymer was selected for further investigation and for micro injection molding.

Fig. 1: QVCD Design (left) and moulded QVCS (right)
Figure 1 shows the QVCD designed for micro injection moulding. A mould was constructed and built in our workshop. QVCDs have been molded with a Babyplast 6/10 V micro injection machine under cleanroom conditions.
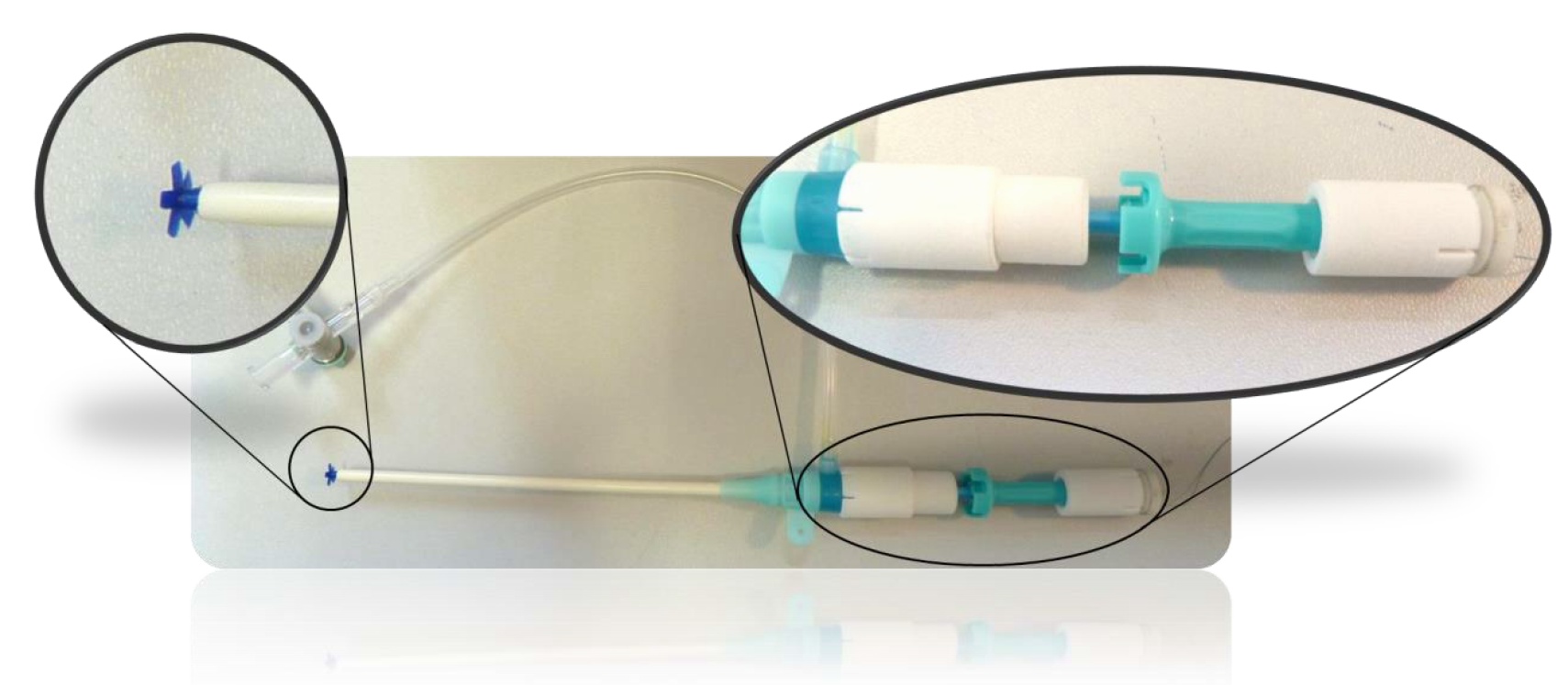
Fig. 2: Application tool with QVCD applied through existing arterial sheath
A tool for the application of the QVCDs through the existing arterial sheath (Radiofocus Introducer II, Fr. 8 used in this example) in situ was designed and produced by rapid prototyping (Figure 2).
The injection moulded QVCDs where tested in a mechanical model by application inside the wall of silicon tubing filled with liquid mimicking an arteriotomy. Biocompatibility of the QVCDs was tested in static and dynamic conditions by incubation with human endothelial cells. Following this in vivo tests have been done in an animal model. QVCDs have been placed in sheep arterial vessels (arteria carotis) following arteriotomy at up to 6 times at diffent places. All 6 animals where mobilized immeditely after anesthesia. No neurological disorders have been observed. After an implantation time of max. 30 days the animals have been sacrificed.
Results and Discussion: The in vivo tests proved that the new QVCD can be placed in the arteriotomy hole through the existing sheath instantly sealing the vessel (Fig. 3). The degradation time of 14 days found in vitro was sufficient for vessel healing. After 30 days the remaining QVCD material was covered by neointima.
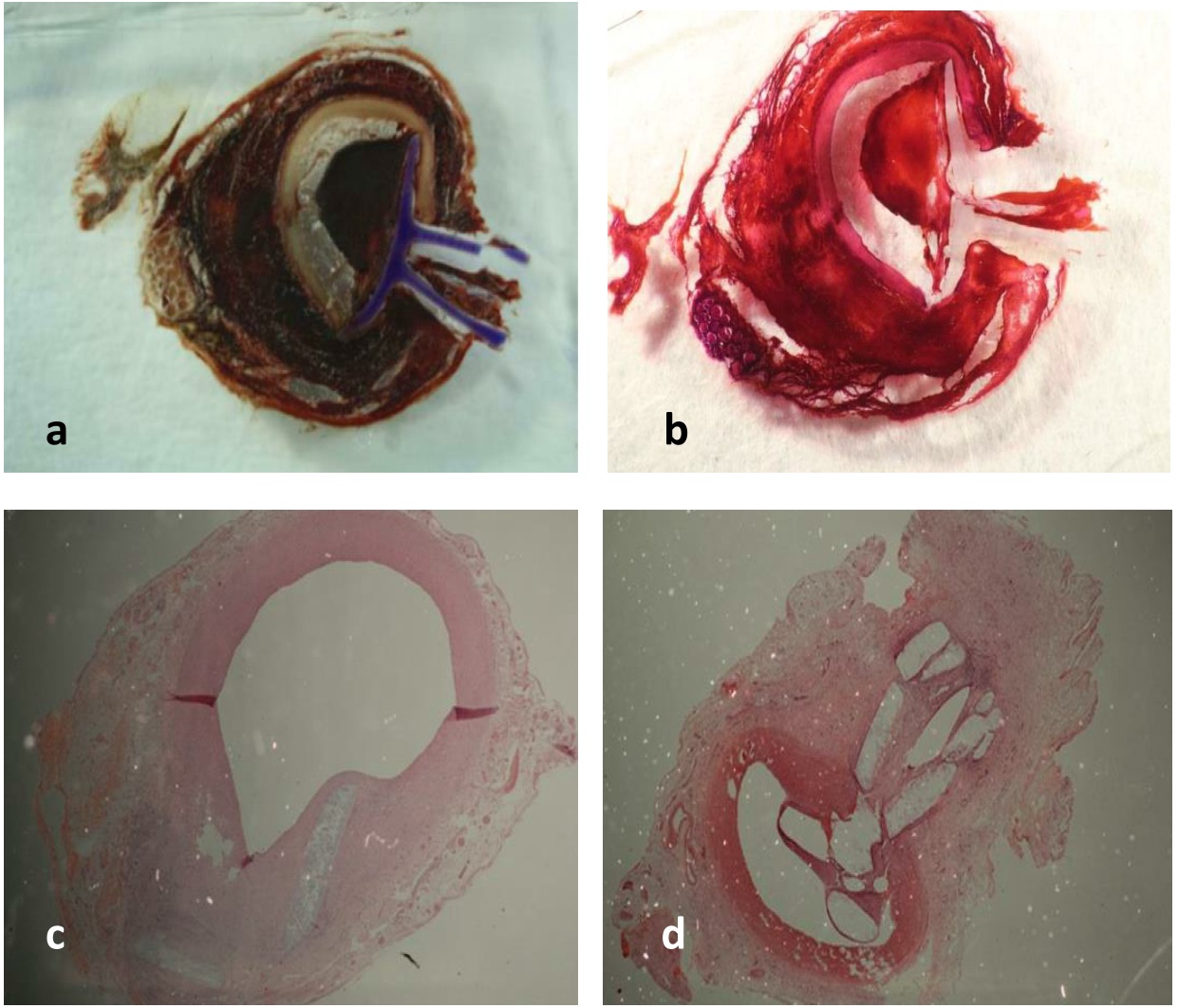
Fig. 3: Histologie (a+b: nativ and HE stained preparation at day one, c+d: HE stained preparation after 30 days of implantation)
No cytotoxic, inflamatory or thrombogenic effects have been found and endothelialisation of the QVCDs could be shown.
Conclusion: The in vivo testing of the new QVCD showed the feasibility of applying the novel vascular closure device through an existing arterial sheath. The in vitro results for degradation and biocompatibility as well as the in vivo findings for 8 Fr in animal model encourage future work with larger calibers.
The authors would like to thank the German Federal Ministry of Education and Research (Grant no: 01KQ0902K-M) for providing financial support to this project in the frame of Gesundheitsregion REGiNA.
References:
[1] Hon L-Q. et al., Curr Probl Diagn Radiol (2009), 38(1):33-43
[2] Venkatesan D. et al; JACC: Cardiovascular Interventions (2012) 5, 8: 837–844
[3] Wendel H-P, Neumann B: (2010) EP 1648 308 B1