Introduction: Biocompatibility evaluation is a key scientific task in the study of biomaterials. The newly developed systems biology which consists of multi-biomics becomes a powerful tool to explain the mechanism of the interaction between biomaterials and the human body. This study uses multi-biomics technologies (genomics, proteomics, microRNA sequencing, metabonomics, etc) in combination with bioinformatics analysis to illustrate the mechanism of interaction between 10 kinds of materials (nickel ion, gold/silver nanoparticles (GNPs/SNPs), hydroxyapatite (HA), bare and nickel titanium (NiTi) alloy coated titanium nitride (TiN), Poly(L-lactic acid) (PLLA) nanofibers) and differents cells from the perspective of system biology.
Materials and Nethods: 10 kinds of materials were prepared and L929, human dermal fibroblast, mouse bone marrow mesenchymal stem cells, human umbilical vein endothelial cells and PC12 cells were cultured respectively. MTT, CCK8 assay, flow cytometry were applied for evaluating the influence of materials on cell proliferation, cell cycle and apoptosis. Gene expression profile microarray, proteomics, microRNA sequencing and metabonomics technologies were used to screen the differentially expressed mRNA, protein, microRNA and metabolite. Clustering analysis, functional classification and biological pathway analysis were carried out, and then integrative analysis of multi-biomics data was performed to filter key biological pathways and involved key mRNA, protein, microRNA and metabolite. Various cellular and molecular biology methods were further introduced to verify the key biological pathways, and finally the molecular mechanism of material-cell interaction were discussed by combining the results of cytology, biomics and bioinformatics analysis[1]-[5].
Results and Discussion: Nickel ion might affect extracellular matrix, glucose transport and cytoskeleton, lead to oxidative damage and possible DNA damage, induce changes in protein synthesis and energy metabolism and finally produce cytotoxicity (Fig. 1 is the phenylalanine metabolism pathway affected by nickel ion). GNPs affected the cell cycle but inhibited apoptosis and showed good cell compatibility; while SNPs destroyed cytoskeleton, inhibited ATP synthesis, induced apoptosis, and ultimately leading to cytotoxicity. HA could mediate cell proliferation, morphology and differentiation through several biological pathways related to cell cycle and proliferation, cytoskeleton and cell adhesion, as well as the multi-function and differentiation of stem cells. Among them, MAPK pathway played a major role in osteogenic induction of natural hydroxyapatite. Bare NiTi alloy inhibited the function of endothelial cell in phase of growth and proliferation at molecular level for the increasing release of nickel ion. However, TiN coating could not only effectively prevent the release of nickel ion from NiTi alloy, but also improve endothelial cell function by promoting actin cytoskeleton organization and focal adhesion formation, enhancing energy metabolism, increasing regulation of inflammatory response, and inhibiting apoptosis. Aligned PLLA nanofibers might promote PC12 cells differentiation via amino acid metabolism and lipid metabolism pathway, in which phenylalanine, norepinephrine and arachidonic acid might play crirical roles (Fig. 2).
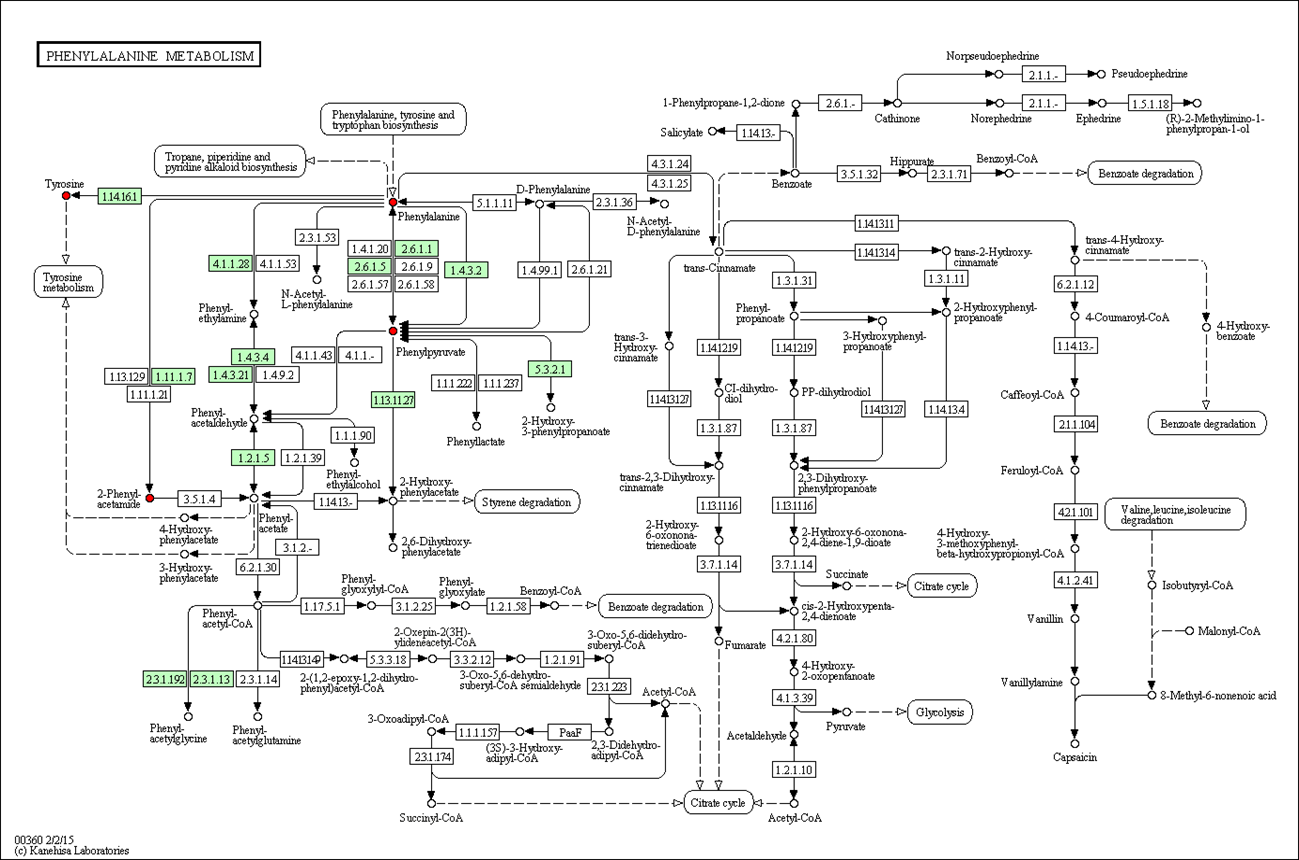
Fig.1 Affected phenylalanine metabolism pathway by nickel ion-induced differentially expressed metabolites in L929 cells.
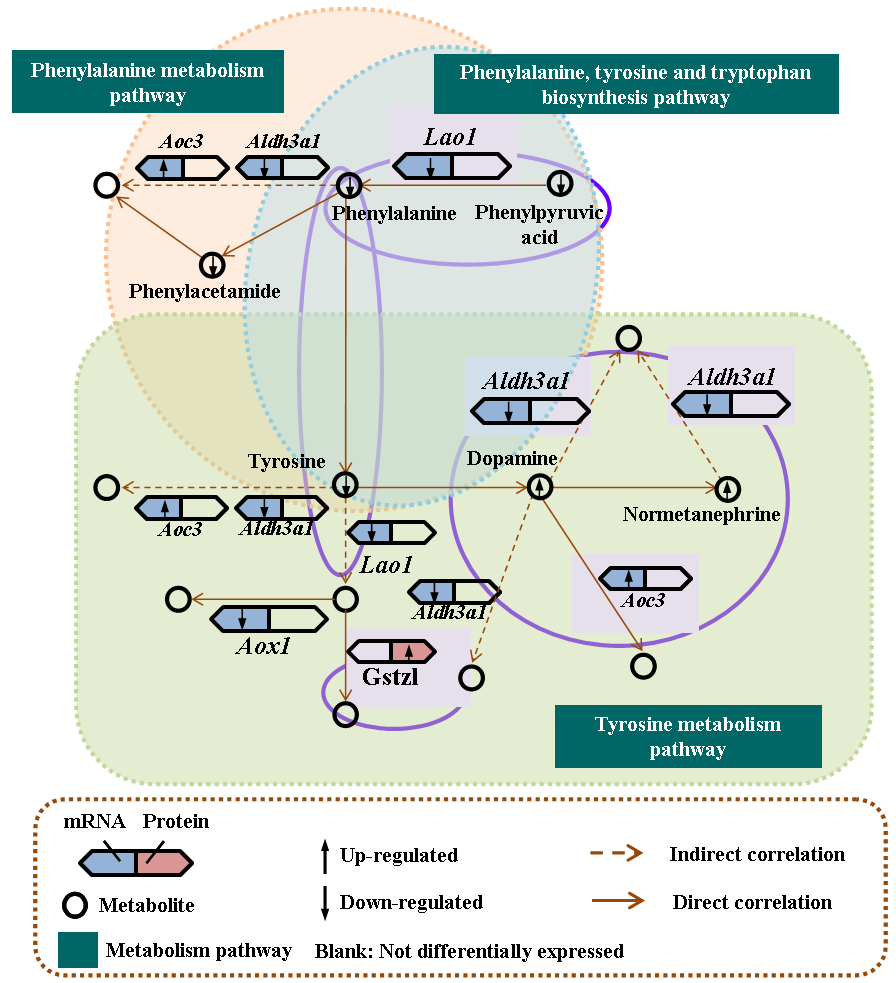
Fig. 2 The influence of aligned PLLA nanofibers on PC12 cells differentiation via amino acid metabolism pathways.
Conclusion: By combining all multi-biomics technologies contained in systems biology for the first time, our group illustrated the mechanism of biomaterial-cell interaction at the molecular level sensitively, efficiently and comprehensively. This technical route can also be used to study the interaction mechanism of other biomaterials on human body. It also lays a foundation for exploring the integrative analytical model of information biology and systems biology.
National Nature Science Foundation of China(No.31271012, 31170910, 30470478, 30770583); 973 project (No. 2009CB930000); The Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20100092110027); Natural Science Foundation of Jiangsu Province of China (No. BK2007109)
References:
[1] Xiaoying Lü, Huiqin Lu, Lifeng Zhao, et al. Genome-wide pathways analysis of nickel ion-induced differential genes expression in fibroblasts. Biomaterials, 2010, 31,1965-1973.
[2] Yan Huang, Xiaoying Lü, Yinghua Qu, et al. MicroRNA sequencing and molecular mechanisms analysis of the effects of gold nanoparticles on human dermal fibroblasts. Biomaterials, 2015,37, 13-24.
[3] Dayun Yang, Xiaoying Lü, Ying Hong, et al. The molecular mechanism for effects of TiN coating on NiTi alloy on endothelial cell function. Biomaterials, 2014, 35,6195-6205.
[4] Xiaoying Lü, Jiandan Wang, Bin Li, et al. Gene expression profile study on osteoinductive effect of natural hydroxyapatite. Journal of Biomedical Materials Research: Part A, 2014, 102,2833-2841
[5] Yadong Yu, Xiaoying Lü, Fei Ding. Influence of Poly(L-Lactic Acid) Aligned Nanofibers on PC12 Differentiation. Journal of Biomedical Nanotechnology, 2015,11, 816-827.