In 2004, scientists in Clemson University of America created firstly a type of new carbon nanomaterial-fluorescent carbon dots. Compared with other fluorescent nanomaterials, fluorescent carbon dots have a variety of advantages such as a wide range of raw materials, simple preparation process, low biological toxicity, good biocompatibility and excitation wavelength-dependent photoluminescence, etc[1]. Therefore, fluorescent carbon dots have attracted more and more attention in many biomedical applications including biological probe, bio-imaging, cell labeling, biosensor, disease diagnosis, and drug delivery, etc[2]-[3].
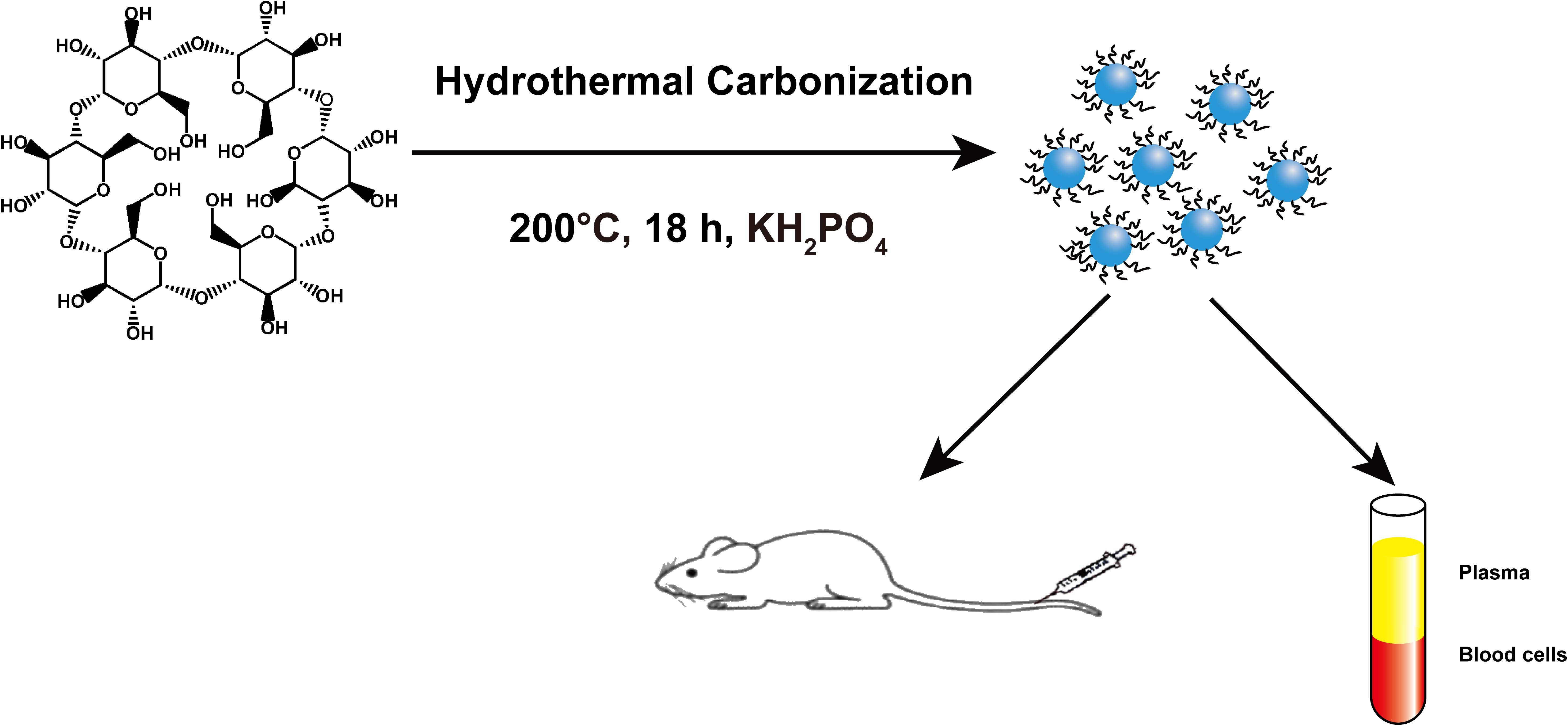
In these biomedical applications, carbon dots will inevitably contact with blood tissue, so it is essential to evaluate in vitro and vivo blood compatibility of fluorescent carbon dots, which will promote the clinical application of fluorescent carbon dots. We prepared fluorescent carbon dots by hydrothermal carbonization of α-cyclodextrin. The fluorescent carbon dots had uniform particle size and strong blue fluorescence, such as Figure. 1. In this work, we investigated the effects of the fluorescent carbon dots on the structure and function of key blood components in vitro and the blood clotting in vivo. The results show that the effect of the fluorescence carbon dots on blood components with concentration dependent. It only had a weak effect on blood components with the fluorescence carbon dots ≤0.1 mg/mL. However, higher concentrations of the fluorescence carbon dots impaired the normal structures and functions of the key blood components in vitro. No blood coagulation and organs damage were found, when the dose of the fluorescence carbon dots ≤50 mg/kg. These findings provide important information for circumventing the potential risks and promoting the clinical applications of the fluorescent carbon dots.
References:
[1] Xu, X.; Ray, R.; Gu, Y.; Ploehn, H. J.; Gearheart, L.; Raker, K.; Scrivens, W. A. Electrophoretic Analysis and Purification of Fuorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736-12737.
[2] Da Silva, J. C. G. E.; Gonçalves, H. M. R. Analytical and Bioanalytical Applications of Carbon Dots. TrAC, Trends Anal Chem. 2011, 30, 1327-1336.
[3] Baker, S. N.; Baker, G. A. Luminescent Carbon Nnodots: Emergent Nanolights. Angew. Chem., Int. Engl. 2010, 49, 6726-6744.