Introduction: Many care receivers, who hardly brushing their tooth enough by themselves, have high risks of intraoral bacterial infections, and following contract pneumonia. Adsorption of salivary proteins on teeth and forming dental plaque causes the infections and control of salivary proteins adsorption on teeth makes the risk of the infections reduce. This study aims that hydroxyapatite (HAp) substrate as tooth model obtains anti-biofouling properties by a simple method for short time. It is well known that the polymers composed of 2-methacryloyloxyethyl phosphorylcholine (MPC) have excellent anti-biofouling properties[1]. Thus, we synthesized well-defined and water-soluble MPC polymers, which can attach the HAp substrate spontaneously from their aqueous solution. That is, we applied a mechanism of mussel adhesion for immobilization of the MPC polymer on HAp surface. This adhesion mechanism does not need any chemical and physical treatment of the teeth substrate, so that it is suitable for intraoral treatments. In addition, the MPC polymers can make crosslinking under slightly alkali condition and the polymer layer may be stable for a long period. Here, we will discuss fundamental properties of the MPC polymers adhered on the HAp.
Materials and Methods: The atom transfer radical polymerization (ATRP) initiator group was applied to make well-defined structure. At first, ATRP initiator was introduced in dopamine. By using this compound, MPC and 2-aminoethyl methacrylate (AEMA) were polymerized under controlling polymerization degree and composition of these monomer units (PMA-Dopa, Fig. 1).
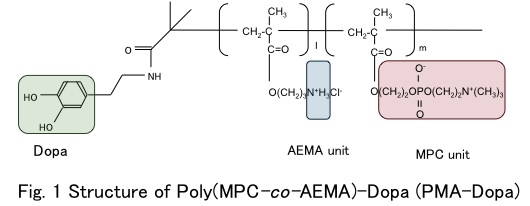
The HAp plate was immersed in the PMA-Dopa solution (PB, pH = 8.0, 20 mg/mL) at 30°C for 1.0 h (PMA-Dopa/HAp). The surfaces of these HAp plates were analyzed by XPS and contact angles of water. Amounts of PMA-Dopa adsorbed on HAp plate for different treatment periods and the amounts of protein adsorbed on the PMA-Dopa/HAp substrates were measured by QCM-D.
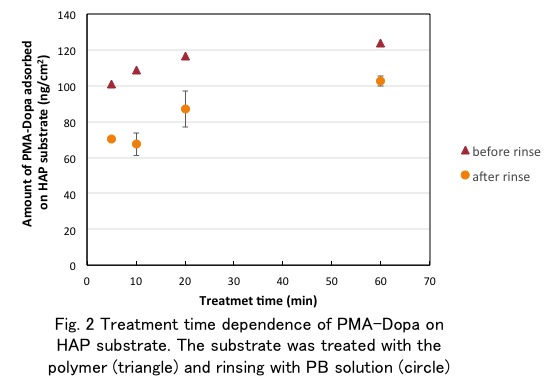
Results: The XPS analysis indicated that the every elements of PMA-Dopa were observed on the PMA-Dopa/HAp. This result means that PMA-Dopa was successfully coated on HAp substrate by a simple procedure. The contact angle of water on PMA-Dopa/HAp was about 14 º and much smaller than that on original HAp (about 66º). As shown in Fig. 2, the amount of PMA-Dopa adsorbed on the HAp increased up to about 120 ng/cm2 for 20 min and even after rinsing, the PMA-Dopa still remain on the HAp surface up to about 90 ng/cm2.
This is due to selective reaction between terminal dihydroxyphenyl group and HAp substrate. Amounts of albumin adsorbed on the PMA-Dopa/HAp substrate were about 30 ng/cm2 while that on original HAp substrate were 125 ng/cm2. The PMA-Dopa coating suppressed protein adsorption on HAp substrate. As a perspective for practical tooth treatments, we are now trying to shorten the treatment time to form stable anti-biofouling MPC polymer layer.
References:
[1] Ishihara, Kazuhiko, et al. "Inhibition of fibroblast cell adhesion on substrate by coating with 2-methacryloyloxyethyl phosphorylcholine polymers." Journal of Biomaterials Science, Polymer Edition 10.10 (1999): 1047-1061.