Introduction: Curcumin is an herbal/nutraceutical supplement with multiple pharmacological activities, which are beneficial in several complex chronic diseases such as Alzheimer’s, arthritis, cancer etc. Despite its demonstrated efficacy, the success of curcumin is yet to be translated because of its poor bioavailability caused by extremely low water solubility, rapid degradation and metabolism.
The current study for the first time reports that Eudragit® EPO a safe polymer forms soluble amorphous complexes with curcumin and thereby, enhances the aqueous solubility and stability of curcumin, and increases its oral bioavailability in mice.
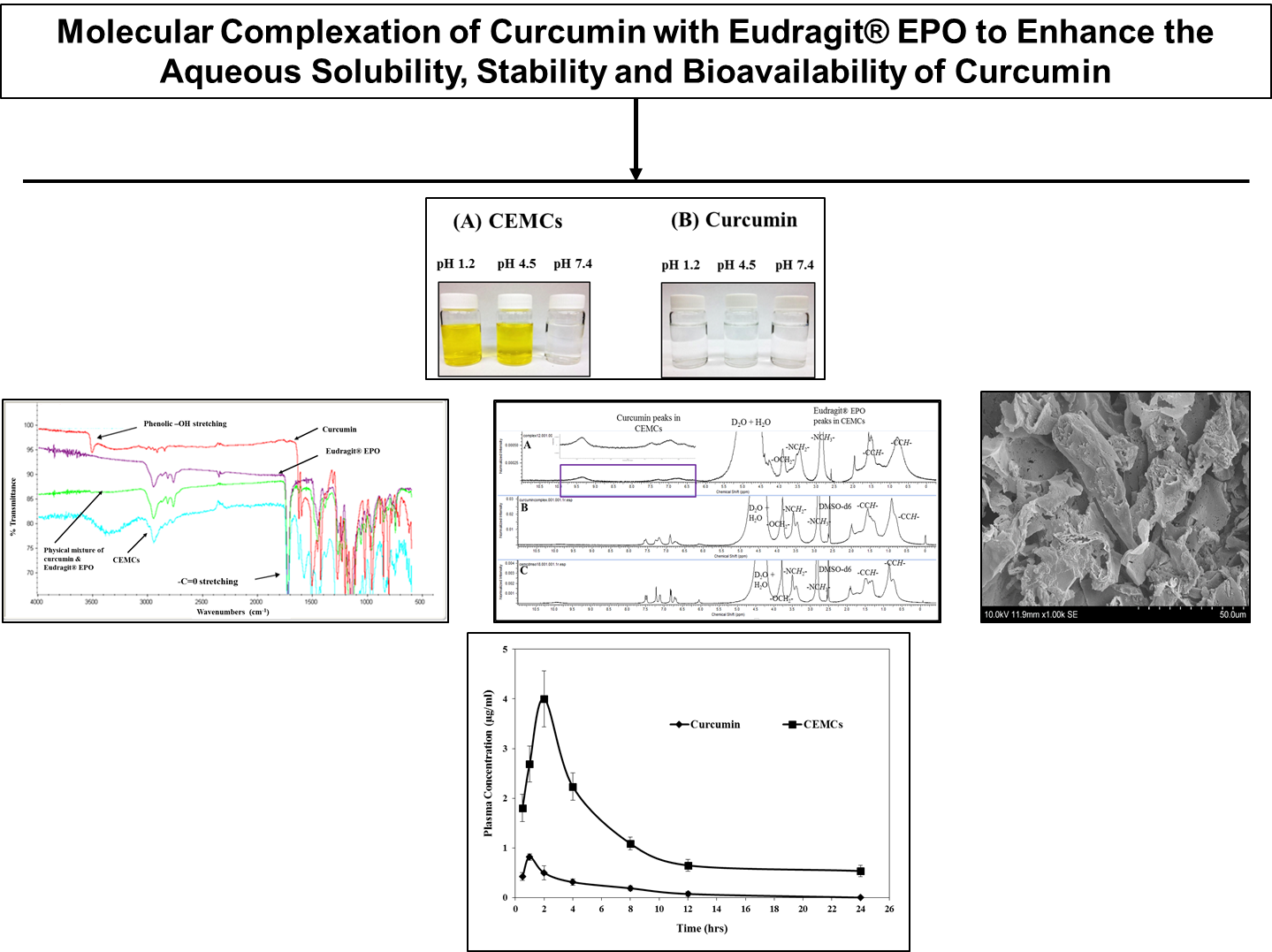
Materials and Methods: CEMCs (Curcumin and Eudragit® EPO molecular complexes) were prepared using nanoprecipitation method. The process of CEMCs formation was optimized to enhance curcumin loading and its aqueous solubility by varying the type of organic solvent (acetone, ethyl acetate, and ethanol), surfactants (tween-20, pluronic F-68, and polyvinyl alcohol (PVA) and ratio of curcumin to Eudragit® EPO used in preparation of the molecular complexes (1:2, 1:3 and 1:5 of curcumin respectively per 100 mg of Eudragit® EPO). To determine the apparent solubility of curcumin, unformulated curcumin or equivalent amount of CEMCs were dispersed in pH 1.2 solution (0.1N HCl) and incubated at 37 °C for 4 h with 100 rpm shaking. The concentration of soluble curcumin was measured by UV absorbance at 420 nm. Further, the bioavailability of curcumin was assessed after oral administration in mice (150 mg/kg). The plasma concentration of curcumin at different time intervals after ingestion was detected by RP-HPLC with UV absorption at 420 nm
Results and Discussion: With the preliminary novel technology described in the current piece of work, Eudragit® EPO formed soluble amorphous complexes with curcumin (CEMCs). This enhanced the aqueous solubility of curcumin from ~1 µg/mL to ~20 mg/mL. Acetone and polyvinyl alcohol as solvent and surfactant respectively, improved the curcumin loading and its apparent solubility. Curcumin loading was further improved with the increasing the drug-polymer ratio to 1:2. CEMCs are highly soluble in buffers at gastric pH and are more stable in buffers at a range of gastro-intestinal pHs compared to unformulated curcumin. CEMCs also enhanced the oral bioavailability of curumin in mice by several folds (AUC; ~20 times and Cmax; ~6 times).
Conclusion: The present study is based on the preparation of a novel formulation that potentially enhances the oral bioavailability of curcumin by increasing its aqueous solubility and stability. This is the first study reporting such a highly soluble curcumin formulation for oral delivery purposes. The CEMCs has several competitive advantages over existing technologies: high aqueous solubility and loading, stable at wide range of pHs, cost effective and use of FDA approved ingredients. This is a proof of concept study that has great potential to enhance the therapeutic efficiency of curcumin in various complex chronic diseases.
Authors are thankful to the Department of Pharmaceutical Sciences, South Dakota State University; Research support funds from the office of research, SDSU; Technology transfer office (TTO), SDSU; Evonik Industries