Introduction: Scanning electron microscopy (SEM) at the taper junction of retrieved hip prostheses has shown characteristic pits that cannot be attributed to mechanical wear processes. Furthermore histological examination of inflammatory peri-prosthetic tissue in failed metal-on-metal hip replacements has demonstrated the presence of mononuclear and macrophage cells. We propose that differentiated osteoclasts play a key role in biological corrosion of metal surfaces [1][2][3].
Methods: Mononuclear cells from the buffy layer of ovine blood were harvested and seeded onto medical grade (<3Ra) discs of stainless steel, cobalt chrome and titanium. 100,000 seeded cells per disc were cultured for 21 days using a medium containing RANK-L and M-CSF. Culture supernatant was assessed using a TRAP assay and discs were imaged using SEM. In order to determine the resorption potential of these cells they were also grown on bone surfaces and the morphology of the resorption pits assessed.
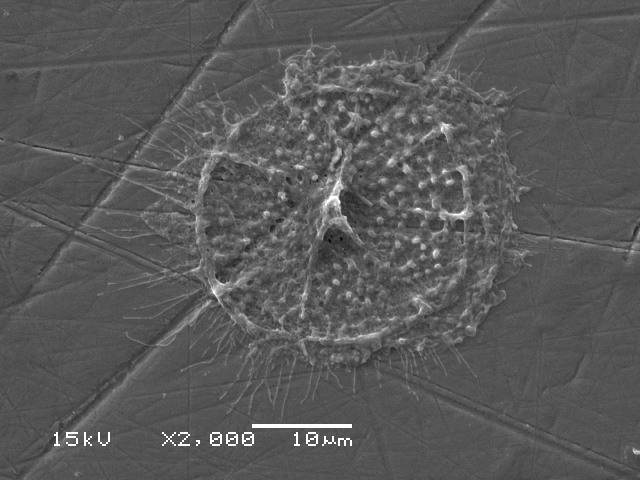
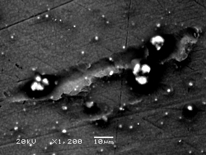
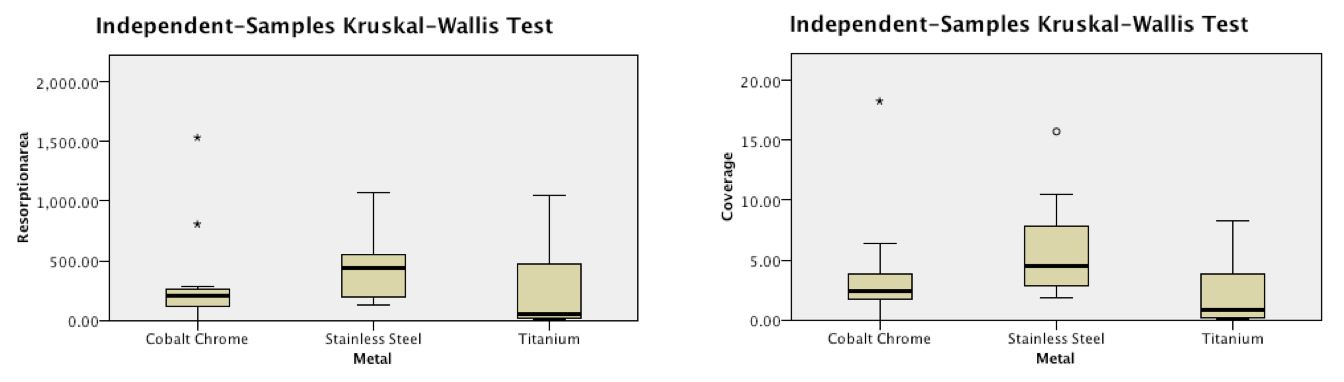
Results: Qualitative SEM analysis demonstrated characteristic cellular appearances of mature osteoclasts on all three metal surfaces. These cells showed evidence of movement and resultant corrosion pits were seen on the alloy surfaces. These pits measured around 250um in width and were similar is shape to those seen on bone surfaces treated with these cells. Quantitative analysis demonstrated peak levels of TRAP at 7 days on all three surfaces consistent with the presence of differentiated osteoclasts. Stainless steel showed a significantly greater number of osteoclast attached, cellular coverage and more numerous resorption pits covering a greater area.
Conclusion: Osteoclastic bio-corrosion in total hip arthroplasty may be important leading to the release of toxic metal ions such as chromium and cobalt. Peripheral mononuclear cells can be successfully differentiated into osteoclasts when seeded onto medical grade metal alloys. These osteoclasts produce TRAP and exhibit evidence of corrosion pits and migration on the metal surface. Future work aims to demonstrate that osteoclasts lower the electrochemical potential required for fretting corrosion to occur.
RANK-L : Receptor Activator for Nuclear Factor K B Ligand
M-CSF: :Macrophage Colony Stimulating Factor
TRAP : Tartrate Resistant Acid Phosphatase
References:
[1] CADOSCH, D., AL-MUSHAIQRI, M. S., GAUTSCHI, O. P., MEAGHER, J., SIMMEN, H.-P. & FILGUEIRA, L. 2010. Biocorrosion and uptake of titanium by human osteoclasts. Journal of Biomedical Materials Research Part A, 95A, 1004-1010.
[2] CADOSCH, D., CHAN, E., GAUTSCHI, O. P. & FILGUEIRA, L. 2009. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening-Current concepts. Journal of Biomedical Materials Research Part A, 91A, 1252-1262.
[3] CADOSCH, D., CHAN, E., GAUTSCHI, O. P., SIMMEN, H.-P. & FILGUEIRA, L. 2009. Bio-Corrosion of Stainless Steel by Osteoclasts-In Vitro Evidence. Journal of Orthopaedic Research, 27, 841-846.