Introduction: Collagen remodeling is an integral part of tissue development, maintenance, and regeneration. During remodeling, collagen triple helices are denatured and degraded which exposes single-stranded collagen. Collagen hybridizing peptides (CHPs), also known as collagen mimetic peptides (CMPs) have been shown to bind specifically to denatured collagen through triple helical hybridization[1]. CHPs require peptide sequences with strong propensities triple helical folding such as Gly-Pro-Hyp (GPO) repeats or peptoid-containing sequences such as Gly-(N-Ile-Gly)-Pro[2]. Current CHPs are limited by their slow binding rate and need for heat or photo-trigger before use. In addition, the ability to not only trigger binding, but also induce unfolding and unbinding from denatured collagen would be useful for many translational applications in diagnostics, drug delivery, and tissue engineering.
Here, we present a novel peptoid-based CHP which exhibits photo-induced unfolding of its triple helix near physiological temperature and a dimeric CHP which shows improved gelatin binding when compared to monomeric CHP.
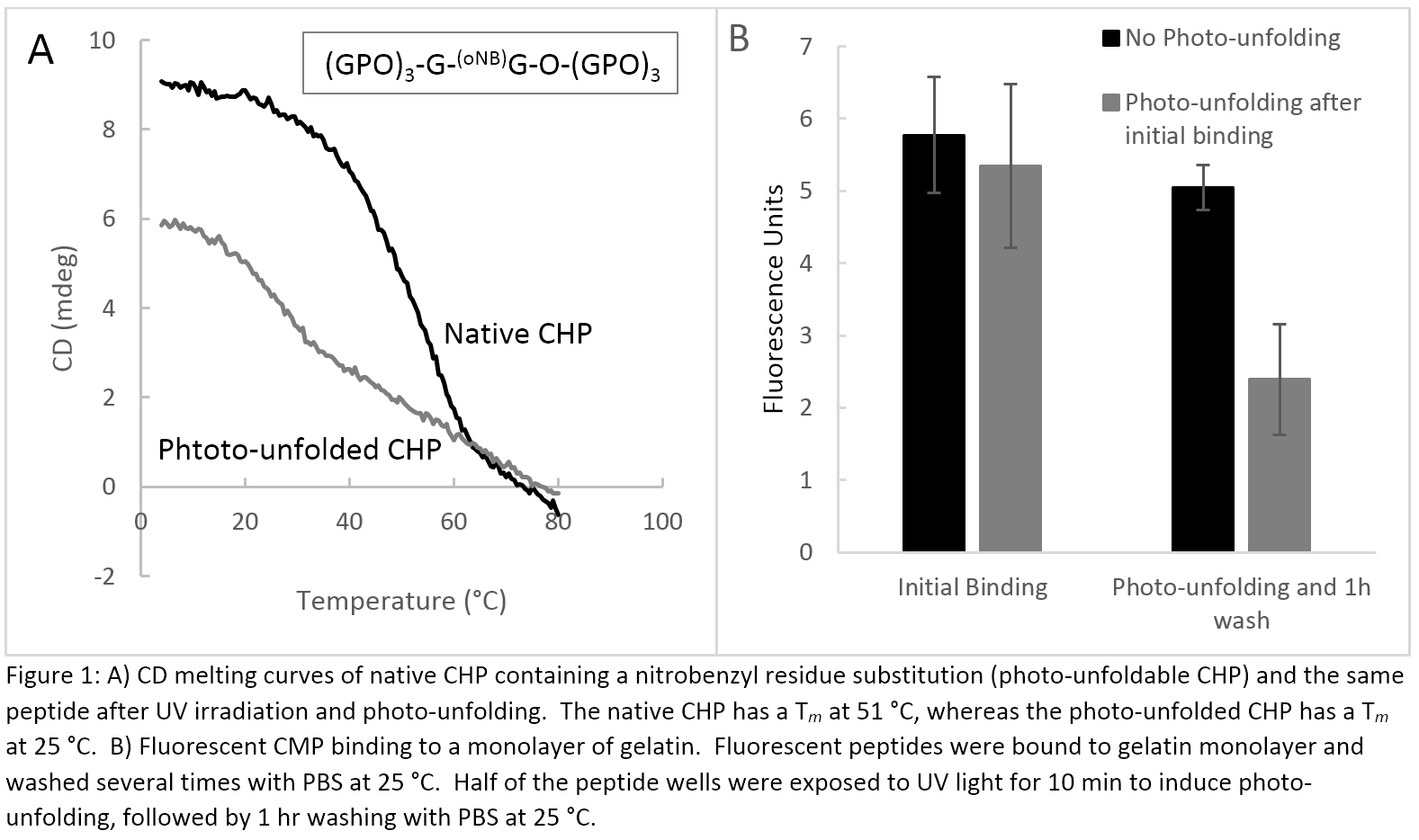
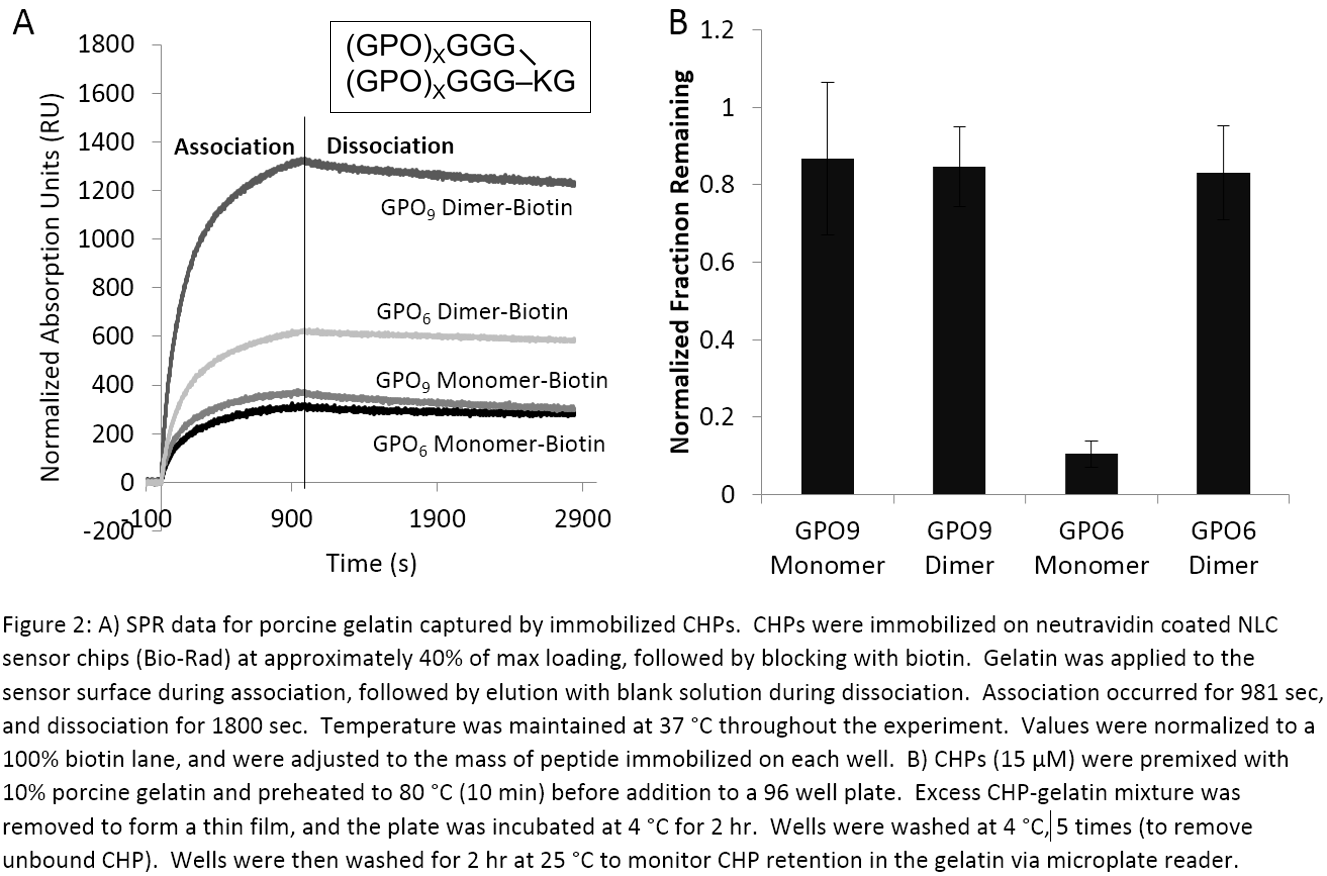
Materials and Methods: Peptides were synthesized using standard, Fmoc-mediated solid phase peptide synthesis. Photo-reactive amino acids were added to the peptoid-based CHP (inset, figure 1A) through substitution of proline in the G-P-O sequence with N-oNB-Gly, which converts to Gly after exposure to UV light (inset, figure 1A). Dimers (inset, Figure 2A) were created using Fmoc-Lys(Fmoc)-OH to form the branch point. Thermal melting transitions were observed by circular dichroism. Gelatin binding affinity was determined through fluorescence binding assay, as reported[3].
Results and Discussion: Photo-reactive, peptoid CHPs containing oNB-Gly substitutions at the X position in the GlyXY sequence demonstrated a dramatic shift in melting temperature after exposure to UV light (Figure 1A). UV light cleaves the oNB group, converting N-oNB-Gly to Gly which destabilizes the triple helix, resulting in spontaneous unfolding of the CHP, as evidenced by a drastic drop in Tm measured by CD. In a fluorescent binding assay, photo-unfolded CHPs had decreased binding to gelatin and faster release from gelatin monolayers than those not exposed to UV light (Figure 1B). This represents a novel way to induce triple helical unfolding and release of CHP from denatured collagen which could be explored for triggered destruction of nanoscale assemblies based on triple helical folding or delivery of therapeutic CHP derivatives.
Dimeric CHPs refold much faster and have higher binding affinity to gelatin than monomeric CHPs. In SPR, dimeric CHPs immobilized to a sensor chip trapped a larger amount of gelatin from solution than monomeric CHPs at physiologic temperature (Figure 2A). CHP affinity to denatured collagen is typically proportional to the number of GPO repeats and melting point. However, in a fluorescent binding assay, dimeric (GPO)6 had a similar affinity to gelatin as monomeric and dimeric (GPO)9 even with fewer repeats and a lower Tm (Figure 2B). Decreased Tm allows dimeric (GPO)6 to bind denatured collagen at physiologic temperature without any heat activation, which is one of the major limitations of current CHPs.
Conclusions: We have demonstrated two novel CHP structures that can alleviate limitations of current CHPs. The novel, photo-reactive CHP folds into stable triple helix, but spontaneously unfolds after exposure to UV light. This may enable triggered trelease of CHP derivatives from gelatin scaffolds for applications in drug delivery. It can also be used for spatio-temporal localization of CHP in 3D gelatin scaffolds and triggered assembly and disassembly of CHP-mediated nanoconstructs. We also demonstrated improved folding kinetics and high binding affinity for a dimeric CHP which can hybridize to denatured collagen without preheating. We believe that these new CHP architectures will help the development of new imaging and drug delivery systems for diseases associated with high collagen remodeling activity.
Work was supported by grants from NIAMS/NIH (R01-AR060484 and R21-AR065124) and DOD (W81XWH-12-0555) awarded to S.M.Y. and by the Wayne Brown Fellowship, University of Utah, awarded to J.L.K; We would like to thank the other members of the Yu group, Yang Li, Boi Hoa San, Hendra Wayhudi, Bum Jin Kim, Lucas Bennink, Amanda Reynolds, and Jeongmin Hwang for all of their help and assistance.
References:
[1] Li Y, Ho D, Meng H, et al. Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconjug Chem. 2013;24(1):9-16.
[2] Feng Y, Melacini G, Goodman M. Collagen-based structures containing the peptoid residue N-isobutylglycine (Nleu): synthesis and biophysical studies of Gly-Nleu-Pro sequences by circular dichroism and optical rotation. Biochemistry. 1997;36(29):8716-8724.
[3] Li Y, Foss C a., Summerfield DD, et al. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci. 2012;109(37):14767-14772.