Glioblastoma multiforme (GBM), which is the most common and aggressive malignant primary brain tumor with low median survival rate about 15 months on average[1]. Remained infiltrative tumor cells are mainly involved in high recurrence rate. They destroy the extracellular matrix components by infiltration[2]. In this process, glioma cancer stem cells (GSCs) are highly involved. The conventional cancer therapies such as radiotherapy and chemotherapy are futile attempts to inhibit the vigorous infiltration of GSCs because of their resistance against to these therapies[3],[4]. Because of their resistance to conventional cancer therapy, new strategies have been studied to restrain their destructive infiltration.
Hyperthermia is one of the efficient methods to attack tumor cells and reduce migration and invasion as well as degeneration of brain tissues. It is expected to block the invasion of tumor cells below the threshold for coagulation and denaturation of proteins with intracellular heat stress, around 41–47°C[5]. In this approach, nanoparticles could be used to make specific and effective target on brain tumor tissues and enhance of efficacy of drug delivery. In particular, shape-controlled gold nanorod (GNR) is well known to have photothermal effect with near-infrared region (NIR; 650–900 nm) laser[6]. It is relatively simple and well-established nanoparticle with various aspect ratios, enabling tunable absorption of wavelength. Furthermore, it can be kept from aggregating in serum once injected with long circulation time after polyethylene glycol (PEG) treatment on surface[7].
Here, we investigated the effect of GNR-mediated photothermal treatment on the invasiveness of patient-derived glioma cells. We synthesized PEG-protected, CGKRK peptide and Alexa 555-conjugated GNR that exhibit the property of photothermal heat generation under NIR spectrum as well as tumor targetability. To perform systemically, we used a three-dimensional (3D) tumor model consisting of collagen-hyaluronic acid (HA) hydrogel tumor sphere (figure 1A), which mimic the physiologically relevant brain tumor microenvironment where highly express HA in the brain tumor tissue[8]. In our experiment, we optimized the concentration of GNR and duration time of NIR to minimize the cytotoxicity of glioma cells (figure 1C, 1D).
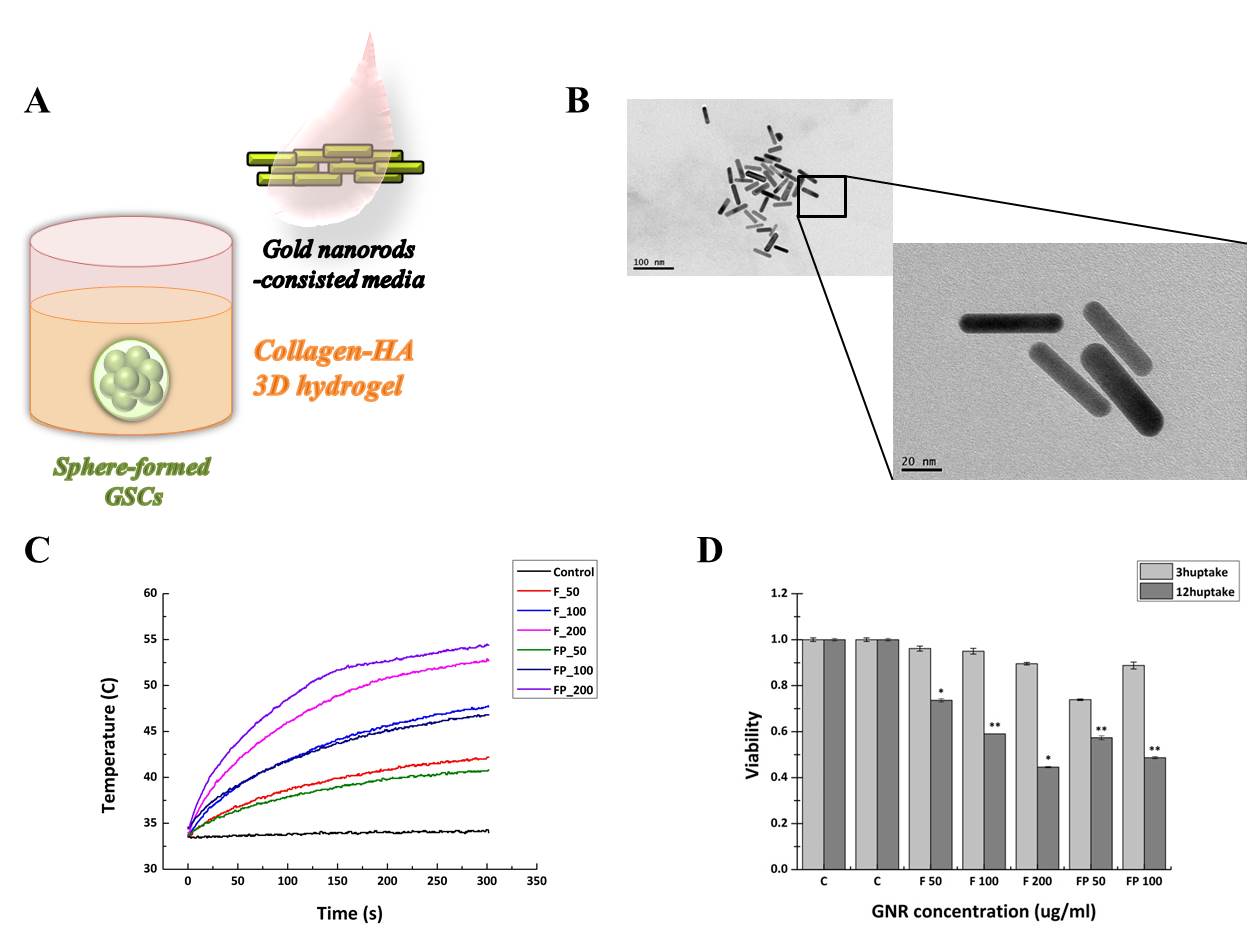
Figure 1. (A) Schematic diagram of sphere formed glioma cancer stem cells (GSCs)-embedded collagen-hyaluronic acid (HA) hydrogel with gold nanorod (GNR) treatment. (B) Scanning electron microscope (SEM) image of fabricated GNR. Size is in 60nm wide with aspect ratio 4:1. (C) Temperature rise by time according to concentration of GNR treatment and GNR type whether targeting peptide attached (FP) or not (F). Generally higher temperature rise is shown with targeting peptide attached GNR. (D) Cell viability test by concentration of GNR treatment within GNR-uptake time in GSCs. More uptake time and higher GNR concentration induce less cell viability.
We treated GNR 10-200 μg/ml for less than 12 hours. We demonstrated the induction of cell death by temperature rise (figure 2A) and inhibition of invasion of GSCs by GNR treatment with NIR laser (figure 2B). Therefore, we could propose a simple but more effective therapeutic approach to target and locally heat GSCs except on normal cells with multi-functional gold nanorods.
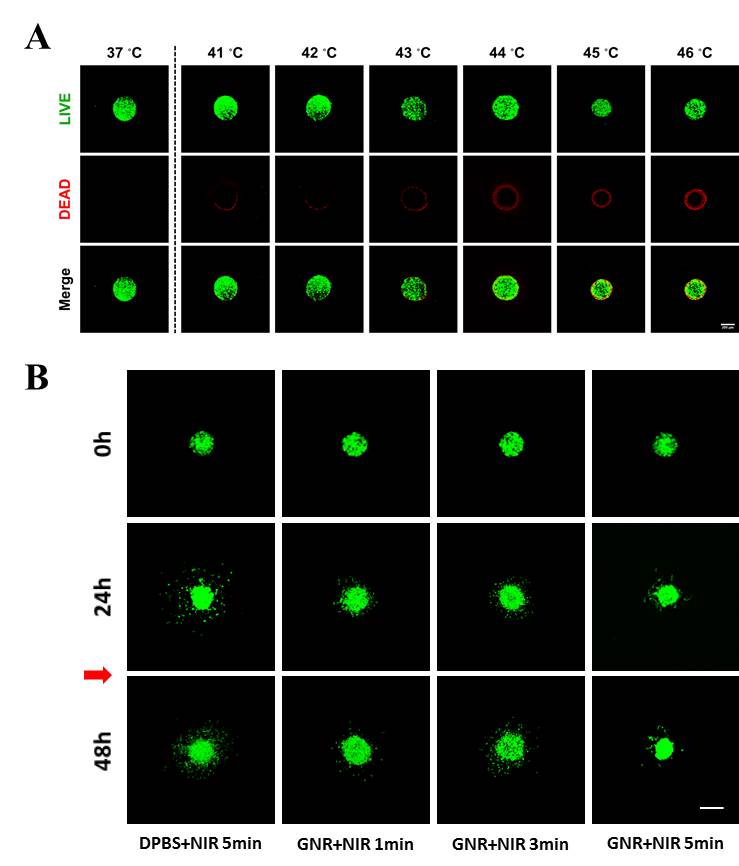
Figure 2. (A) Cytotoxicity of GSCs by temperature growth without GNR. It is shown surface-death of cells by increasing temperature. Scale bar=100μm (B) Inhibition of GSC invasion with GNR. Cells treated GNR with NIR laser (red arrow) shows inhibition of invasion compared DPBS treated control. Scale bar=100μm.
Korea Basic Science Institute (KBSI)
References:
[1] Jeremy N. Rich, Darell D. Bigner, Nature Reviews Drug Discovery, 3, 430-446 (2004)
[2] Scherer HJ, J Nerol Psychiatry, 3, 147-77 (1940)
[3] Barbara Ortensi, Matteo Setti, Daniela Osti, Giulianan Pelicci, Stem Cell Research & Therapy, 4, 18 (2013)
[4] Altaner C, Atlas Genet Cytogenet Oncol Haematol, 16, 757-64 (2012)
[5] Lars O. Svaasand, Charles J. Gomer, Elisa Morinelli, Lasers Med Sci, 5, 121-8 (1990)
[6] Xiaohua Huang, Ivan H. El-Sayed, Wei Qian, Mostafa A. El-Sayed, J Am Chem Soc, 128(6), 2115-20 (2006)
[7] Geoffrey von Maltzahn, Ji-Ho Park, Amit Agrawal, Nanda Kishor Bandaru, Sarit K Das, Michael J Sailor, Sangeeta N Bhatia, Cancer Research, 63(9), 3892-900 (2009)
[8] Delpech B, Maingonnat C, Girard N, Chauzy C, Maunoury R, Oliver O, Tayot J, Cretssard P, Eur J Cancer, 29A, 1012-17 (1993)