Microfiber scaffold combined with cell-derived matrices for chondrogenic mesenchymal condensation
-
1
Korea Institute of Science and Technology, Center for Biomaterials, Korea
-
2
Korea Institute of Industrial Technology, Dept of Technical Application, Korea
-
3
KonKuk University, Dept of Veterinary Medicine, Korea
-
4
University of Science and Technology, Dept of Biomedical Engineering, Korea
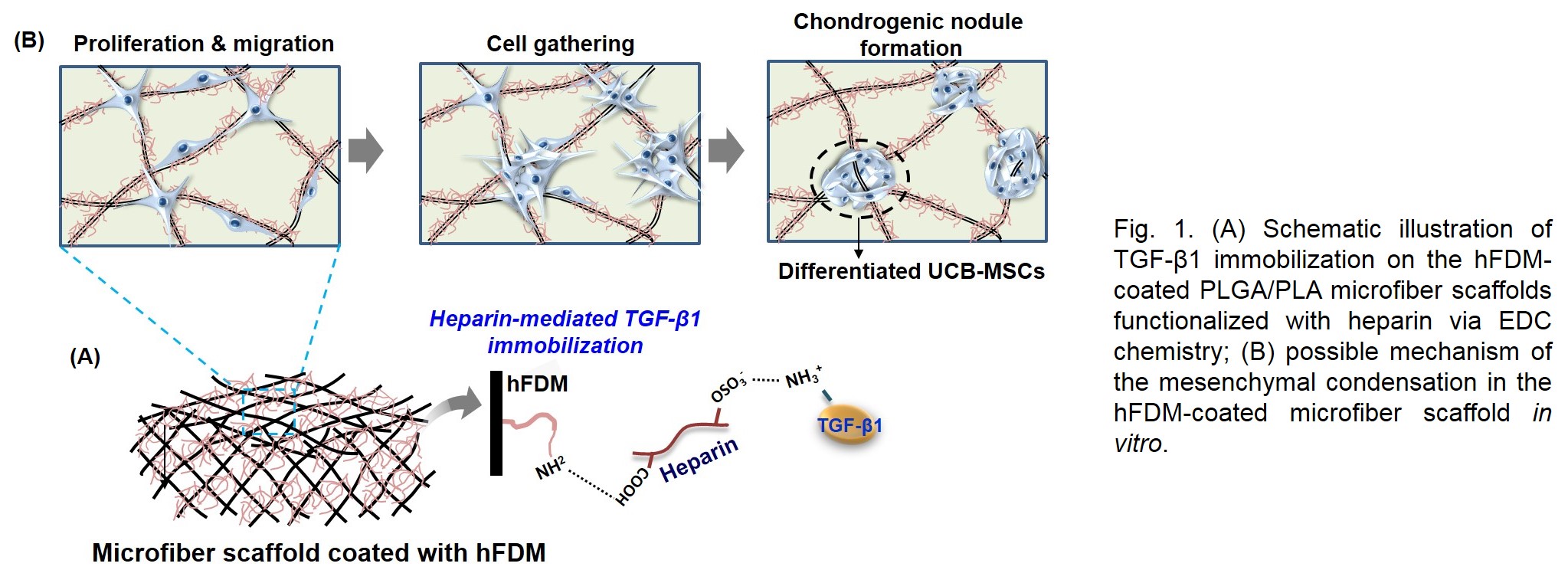
Introduction: Mesenchymal cell condensation has been recognized as a crucial event in chondrogenic development[1]. However current 3D cell culture models used for chondrogenesis pay little attention to the aggregation into a condensed mass of cells. Extracellular matrix (ECM) secreted by cells plays a critical role in cartilage development, by providing a microenvironment to guide cell responses, such as regulation of cell anchorage, migration, organization during chondrogenesis. In this study, a PLGA/PLA based microfiber scaffold was coated with cell-derived extracellular matrix (CDM), then coupled with transforming growth factor-β1 (TGF-β1), and examined for the potential as a mesenchymal condensation-inducible scaffold during chondrogenesis (Fig. 1).
Materials and Methods: Decellularized ECM from in vitro cultured human lung fibroblast (hFDM) was treated onto the surface of PLGA/PLA microfiber scaffold. Heparin was then conjugated on the surface of ECM-coated microfiber scaffold using EDC chemistry and TGF-β1 was subsequently loaded onto it[2]. Once, human umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) were seeded, the effect of hFDM-coated microfiber scaffolds on UCB-MSCs migration, mesenchymal condensation and chondrogenic differentiation was investigated for 28 days in vitro. In addition, a partial-thickness cartilage injury in a rabbit model was used to examine the feasibility of current platform to induce chondrogenesis as well as cartilage regeneration.
Results and Discussion: From the in vitro studies, UCM-MSCs spread well and aggregated along the ECM coated microfibers at 7 days. Both hFDM and hFDM/TGF-β1 groups also showed the significant increases in glycosaminoglycan (GAG) synthesis and the upregulation of chondrogenic marker expression at 21 days. After 14 days, these cell aggregates gradually became slightly rugged spheroid, whereas UCB-MSCs on the non-coated microfiber maintained a spindle-shape. Molecular analysis showed that both hFDM and hFDM/TGF-β1 groups had a significantly higher expression levels of cell migration related-markers. Histological findings demonstrated that TGF-β1 tethered microfiber group found much better newly formed neocartilage in the cartilage defect site. They exhibited specific chondrogenic extracellular phenotype as confirmed by the Col-II immunostaining and Safranin O staining, respectively.
Conclusions: This work suggests that current in vitro model system can be a very promising 3D tool in investigating underlying mechanism of mesenchymal condensation steps during chondrogenic development.
References:
[1] Singh P, Schwarzbauer JE. Fibronectin matrix assembly is essential for cell condensation during chondrogenesis. J Cell Sci, 2014, 127(20):4420-4428.
[2] Kim IG, Hwang MP, Du P, et al. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials, 2015, 50:75-86.
Keywords:
stem cell,
3D scaffold,
Polymeric material,
acellullar matrix
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Regenerative medicine: biomaterials for control of tissue induction
Citation:
Kim
I,
Ko
J,
Do
S and
Park
K
(2016). Microfiber scaffold combined with cell-derived matrices for chondrogenic mesenchymal condensation.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01883
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. In Gul Kim, Korea Institute of Science and Technology, Center for Biomaterials, Seoul, Korea, Email1
Dr. Jaehoon Ko, Korea Institute of Industrial Technology, Dept of Technical Application, Gyeonggi, Korea, Email2
Dr. Sun Hee Do, KonKuk University, Dept of Veterinary Medicine, Seoul, Korea, Email3
Dr. Kwideok Park, Korea Institute of Science and Technology, Center for Biomaterials, Seoul, Korea, kpark@kist.re.kr