Introduction: To avoid physicochemical denaturation during encapsulation of proteins within micro-/nano-particles, proteins have been immobilized onto the surface of particles. However, several challenges like limited surface area and low surface reactivity should be overcome to improve loading efficiency of proteins onto carriers. In this study, porous PLGA microspheres (P-MS) were fabricated by double emulsion method using branched polyethyleneimine (bPEI) as a porogen. Surface of particles were coated with polydopamine for stable and facile immobilization of biotherapeutics onto the P-MS, termed as polydopamine coated P-MS (P-pD-MS). Two types of biomolecules, ovalbumin (OVA) and CpG oligonucleotide (ODN), were utilized as a model antigen and as an adjuvant to trigger immune responses. P-pD-MSs coated with OVA and CpG-ODN were treated to RAW264.7 cells to assess intracellular uptake. After treatment of particles, cytokine releases such as TNF-α and IL-6 and cell viability was examined in vitro.
Materials and Methods: The porous poly(lactic-co-glycolic acid) (PLGA; RG503H) microspheres were prepared by water-in-oil-in-water double emulsion method using a 25 kDa bPEI at the PLGA : bPEI weight ratio of 10 : 1. The P-MS were coated with dopamine in Tris buffer (10 mM, pH8.5) for 3 hours with stirring. P-pD-MSs (100 μg) were reacted with OVA (1000 μg) and CpG-ODN (200 μg) for 24 h at room temperature. Using FITC labeled OVA and TAMRA labeled CpG-ODN, The amount of conjugated biomolecules on P-pD-MS were measured quantitatively. After treatment of P-pD-MSs coated with OVA and CpG-ODN (100 μg) to RAW 264.7 cells, intracellular uptake were visualized by confocal microscopy.
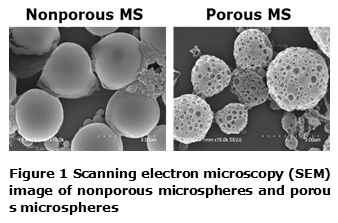
Results and Discussion: The sizes of nonporous and porous particles determined by SEM were 1.60 ± 1.83 and 2.77 ± 2.50 μm, respectively (Figure 1). The pore size was 279 ± 61.6 nm. After surface modification with dopamine, there was no significant change in size and morphology of pores. The amine groups of dopamine were determined by XPS, which suggests that microspheres were successfully coated with polydopamine.
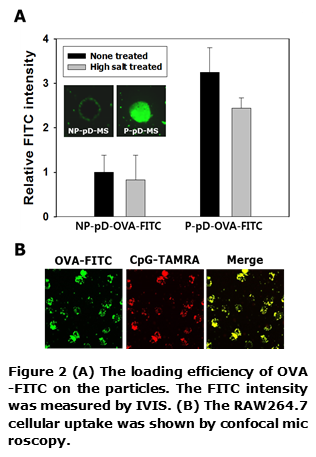
The amounts of FITC-labeled OVA immobilized on the P-pD-MS were higher by ~3-fold than those onto NP-pD-MS, probably due to increased surface area. In addition, conjugated OVAs were not released in high salt condition (Figure 2A). As shown in Figure 2B, P-pD-OVA-CpG-MSs were internalized into RAW 264.7 cells.
Conclusion: In this study, OVA antigen and CpG-ODN adjuvant were successfully conjugated to porous PLGA microspheres via facile polydopamine coating. Fabricated P-pD-OVA-CpG-MS showed excellent intracellular uptake for RAW264.7 cells. Thus, prepared porous and dopamine-coated microparticles could serve as a biocompatible carrier for the delivery of immune-therapeutic antigens and adjuvant.