Introduction: Tendon disorders and injuries are among the most common musculoskeletal problems and their regeneration after injury remains a significant challenge. Given the prevalent fibrous nature of tendons’ ECM, which exhibits an aligned and hierarchical organization in structures from the nano to the macro scale, uniaxial aligned electrospun nanofibers produced from natural/synthetic polymer blends are among the most successful tendon scaffolds in tissue engineering (TE) strategies[1]. These biomaterials can provide the topographical cues to direct cell adhesion and proliferation, as well as positively affecting cell’s differentiation, phenotype maintenance and matrix deposition. However, the limited mechanical properties of electrospun biomaterials restrict their potential application in this field. In the present study, we propose the use of cellulose nanocrystals (CNCs), the “nature carbon nanotubes”, as a strategy for the reinforcement of electrospun poly-ɛ-caprolactone-chitosan (PCL-C) nanofiber scaffolds without compromising their biological performance and thus expand their potential range of application in tendon TE strategies.
Materials and Methods: Random (reference materials) and anisotropically aligned nanocomposite nanofiber scaffolds were fabricated by electrospinning using a static plate or rotating wheel as collecting targets (Fig. 1A), respectively, and their morphology, thermodynamic, and mechanical properties were investigated. The biological performance of human derived tendon cells (TCs) on aligned PCL-C and PCL-C/CNC nanofibers was compared with those of corresponding random nanofibrous materials as composition and topographical controls, assessing their biocompatibility and influence over cell morphology and orientation (Fig. 1B).
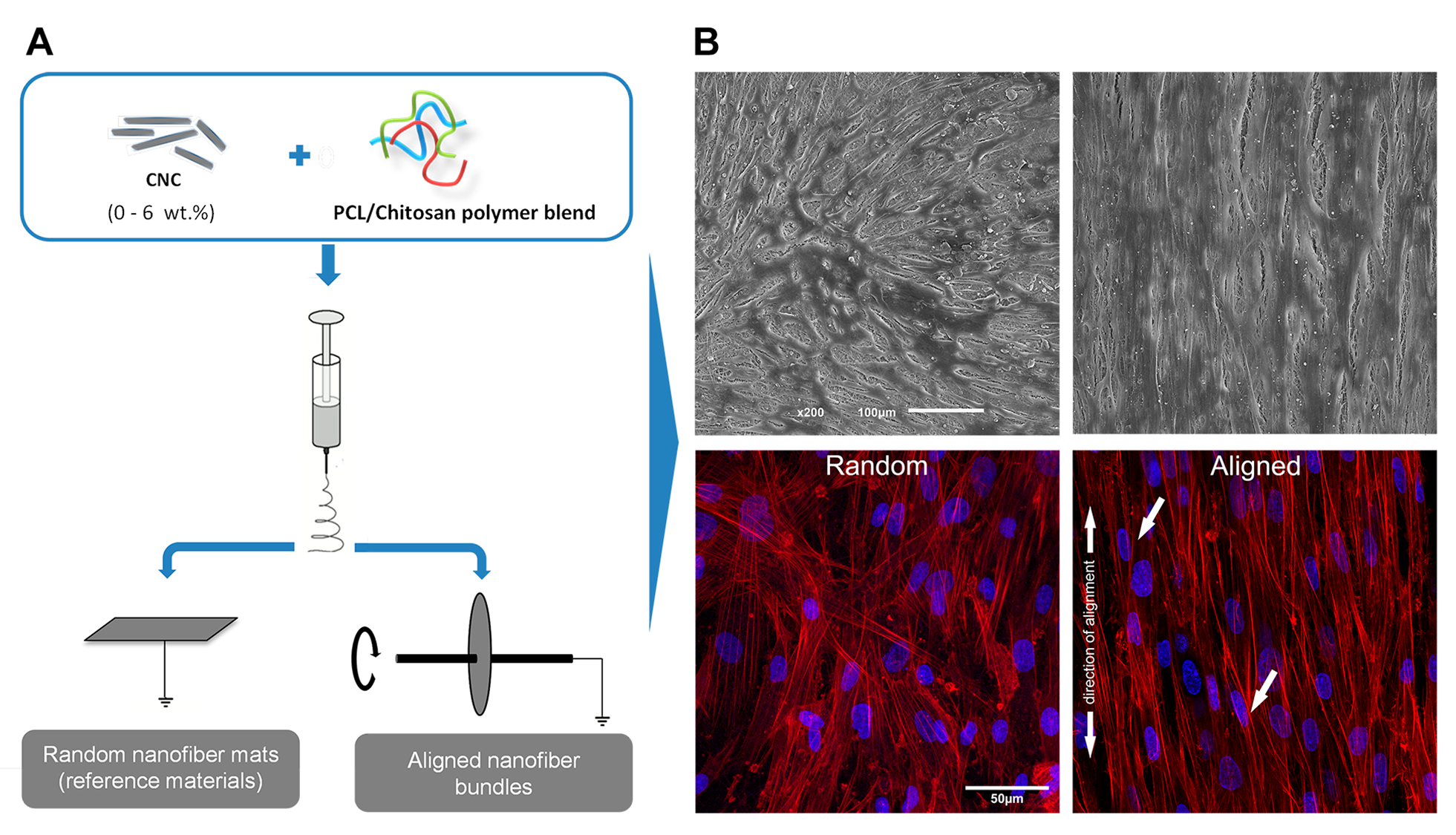
Figure1. A) Schematic representation of the nanocomposite scaffolds fabrication process. B) SEM and confocal microscopy images of TCs seeded on PCL-C scaffolds filled with 3 wt% CNCs. Red: Phalloidin-rhodamine for F-actin; Blue: DAPI for nuclei.
Results and discussion: CNCs were incorporated in PCL-C nanofiber bundles, resembling the typical aligned structure of tendon EMC while raising their mechanical properties up to a tendon meaningful level. In the optimized conditions, the incorporation of 3 wt.% CNCs in the PCL-C nanofiber bundles increased the Young’s modulus to 534 MPa and the tensile strength to 39 MPa, representing an increase of 132% and 83%, respectively, compared to reference PCL-C nanofiber scaffolds, without significantly affecting the biomaterial’s ductility. The incorporation of CNCs in PCL-C nanofibers didn’t affect the cytocompatibility of the biomaterials. Moreover, the aligned nanotopography of the material surface noticeably promoted TCs to orient along the axis of the scaffold through contact guidance mechanisms (Fig. 1B). The observed actin stress fibers demonstrated longitudinal arrangement along the elongated cell shape axis, and their nuclei were evidently more elongated and elliptically shaped as well. These findings are particularly important, as actin cytoskeleton has been shown to play important role on the stage of connective tissue development[2] and elongated cell shape has been demonstrated to be essential for tenocyte function and phenotype maintenance[3].
Conclusions: Our results demonstrate that aligned PCL-C-CNC nanocomposite fibrous scaffolds meet not only the mechanical requirements for tendon TE applications but also provide mimetic ECM topographic cues which are key features for maintaining tendon cell’s morphology and behavior.
The authors also acknowledge the financial support from the Project RL1 - ABMR - NORTE-01-0124-FEDER-000016 cofinanced by North Portugal Regional Operational Programme (ON.2 – O Novo Norte), under the National Strategic Reference Framework (NSRF), through the European Regional Development Fund (ERDF).
References:
[1] Spanoudes K, Gaspar D, Pandit A, Zeugolis DI. The biophysical, biochemical, and biological toolbox for tenogenic phenotype maintenance in vitro. Trends in Biotechnology. 2014; 32:474-82.
[2] Schiele NR, von Flotow F, Tochka ZL, Hockaday LA, Marturano JE, Thibodeau JJ, et al. Actin cytoskeleton contributes to the elastic modulus of embryonic tendon during early development. Journal of Orthopaedic Research. 2015; 33(6):874-81
[3] Zhu J, Li J, Wang B, Zhang WJ, Zhou G, Cao Y, et al. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials. 2010; 31:6952-8.