Introduction: PEEK Optima® has been considered for use as an alternative arthroplasty bearing material due to its biocompatibility and favourable material properties[1]. In this study, the wear of all polymer tibial components was assessed articulating against PEEK Optima femoral components under different environmental conditions; experimental wear simulation of conventional metal-on-polyethylene implant materials was carried out in parallel.
Materials and Methods: Six size C cruciate retaining PEEK Optima femoral components (Invibio Biomaterials Solutions, UK) and six cobalt chrome femorals of similar initial surface topography and geometry were tested against GUR 1020 all polyethylene tibial components (conventional, EO sterilised) in a 6 station ProSim Knee simulator (Simulation Solutions, UK). N=3 of each material type was tested under room temperature conditions as per standard practice at Leeds and n=3 under elevated temperature conditions where the bulk lubricant temperature was maintained at approximately 33°C by incorporation of a heater system into the simulator. The lubricant used was 25% serum supplemented with 0.03% sodium azide and the simulation was run under Leeds high kinematics[2]. 5 million cycles (MC) of wear simulation was carried out under room temperature and 9MC under elevated temperature. The wear of the UHMWPE tibials was assessed by their loss in mass by gravimetric analysis with unloaded soak controls used to compensate for moisture uptake. Statistical analysis was carried out using ANOVA with significance taken at p<0.05
Results: Figure 1 shows the wear of the UHMWPE tibials under the different test conditions. At room temperature, the wear of the UHMWPE tibial was similar against the different femoral materials (p>0.05). Under elevated temperature conditions, the wear rate of UHMWPE was lower than at room temperature for both femoral materials. A high density of light scratches was observed on all the PEEK Optima implants but this did not influence the wear rate which remained constant over the duration of the study.
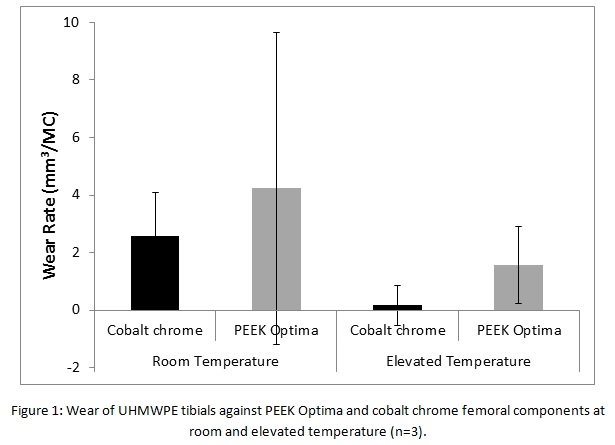
Discussion: The study shows a similar rate of wear of UHMWPE tibials against PEEK Optima and cobalt chrome femorals of similar initial geometry and surface topography independent of the temperature of the bulk lubricant. Testing at an elevated temperature reduced the wear of the UHMWPE against both femoral materials, which may be attributed to deposition of protein from the lubricant onto the articulating surfaces artificially protecting the implants.
Conclusion: This study shows that PEEK Optima has the potential for use as a metal-free knee and that testing under elevated lubricant temperature conditions may have introduced a test artefact which would not occur under room temperature conditions or in vivo.
Invibio Biomaterials Solutions Ltd. provided funding and materials for this study.
References:
[1] Kurtz, S.M. 2007, Biomaterials 28(32):4845-69
[2] McEwen, H.M.J. 2005, J Biomechanics. 38(2):357-365