Introduction: The reconstruction of large bone defects constitutes an important clinical problem, and none of the current approaches were proved to be optimal. Calcium phosphates (CaP) bioceramics have been extensively employed to this purpose. Unfortunately, CaP bioceramics produced from conventional manufacturing processes suffer from sample-to-sample variations or design limitations[1] ; so many detrimental points which prevent to understand the relation between architecture and cell fate, and consequently to improve the ceramic design. In this context, we developed a flexible and robust bioceramics manufacturing process, and investigated the influence of the bioceramic architectural features on the angiogenesis and osteogenesis.
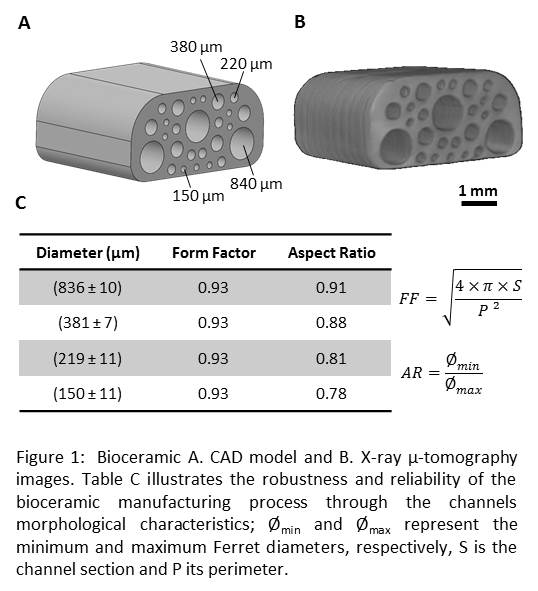
Material and Methods: Bioceramic manufacturing: Bioceramics computer-aided design (CAD) models comprising 4 unidirectional cylindrical pores, were created (Fig. 1). Hydroxyapatite bioceramics manufacturing was carried out by slurry impregnation of an organic template, manufactured from a 3D printer, followed by heat treatments (i.e. debinding, sintering).
In vivo evaluation: Bioceramics were subcutaneously implanted in nude mice up to 30 days for angiogenesis study and 60 days for osteogenesis study. For the latter, bioceramics were coated with rhBMP-2 and/or seeded with human mesenchymal stem cells prior to implantation. Prior to histological analysis, blood vessels, cells nuclei and neo-formed bone were stained with ink, Stevenel’s blue and Van Gieson's picrofuchsin, respectively.
Results: Fig. 1 displays CAD and µ-tomography X images of the ceramics and demonstrates the robustness and reliability of the manufacturing process.
Optical microscope images (Fig. 2) as well as histological evaluation of angiogenesis reveal blood vessel growth inside channels with time, independently to their diameters.More interestingly, even if large blood vessels (Ø > 50 µm) are significantly more numerous in large pores (840 µm), section surface of blood vessels normalized according to channel sections, is constant whatever the channel diameter is. Finally, osteogenic evaluation of rhBMP-2-coated-bioceramics revealed a significant increase in bone formation within the small pores (150 µm).
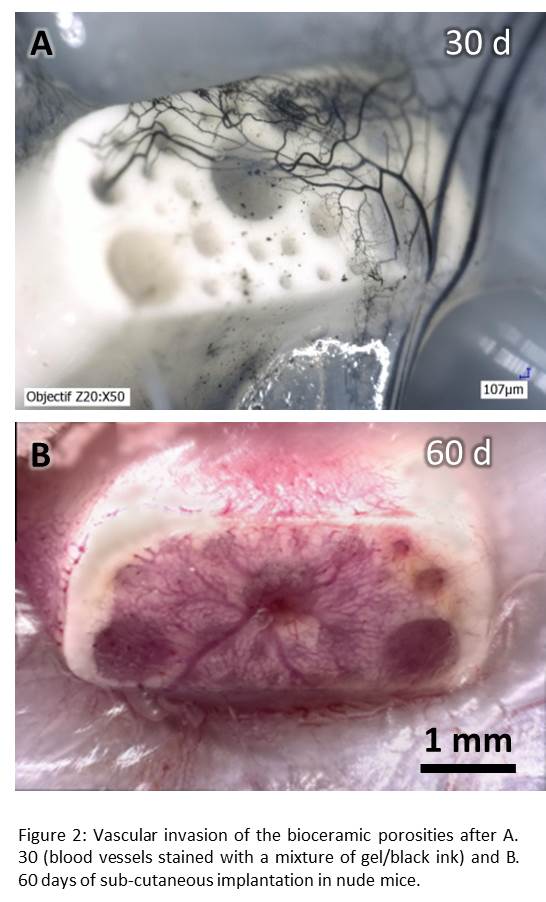
Discussion: These preliminary data revealed a significant effect of the pore size on angiogenesis and osteogenesis. They suggest that large blood vessel (Ø>50µm), which have the capacity to propagate deeper in the scaffold than the capillaries (Ø<25µm), are more numerous in the large pores (840 µm). In opposition, capillaries are significantly more present in small pores (150 µm) where new bone formation occurs preferentially: this would imply that capillaries play a major role in bone ingrowth.
Conclusion: From these results, a strategy to improve efficiently the reconstruction of large bone defects would be to design personalized implants with a gradient of porosity, from large pores at the periphery to small ones at its centre.
References:
[1] Bouet, G., et al., In Vitro Three-Dimensional Bone Tissue Models: From Cells to Controlled and Dynamic Environment. Tissue Engineering Part B: Reviews, 2014