Introduction: Cancer vaccines hold promising potential for cancer imunotherapy due to their systemic action, high specificity, limited toxicity and treatment durability[1]. However, several critical challenges impede realization of cancer vaccines in the clinic. These challenges include inadequate immunological priming[2] and inefficient in-vivo delivery to the lymph nodes[3]. To address these challenges, we set out to develop a new two-component nanovaccine. This nanovaccine is composed of CpG-modified model antigen ovalbumin and anti-CpG-modified iron-oxide nanoparticles; components that are tethered through complementary single stranded CpG and anti-CpG DNA. We hypothesized that the covalent modification of the model antigen ovalbumin (OVA) with the immunostimulatory adjuvant CpG would improve immunological priming[4], while tethering to iron-oxide nanoparticles (NP) would serve as a lymph node delivery vehicle[5]. Herein, we present evidence for the formation and in-vitro functionality of OVA-CpG/anti-CpG-NP nanocomplexes and initial proof-of-concept delivery in-vivo.
Materials and Methods: The model nanovaccine was formulated in three stages as depicted in Figure 1. OVA-CpG conjugates were analyzed with anion exchange chromatography and SYBR Gold and BCA quantitative spectrophotometric assays for single stranded DNA (ssDNA) and protein, respectively. Complexation between conjugates and iron-oxide nanoparticles was verified with agarose gel electrophoresis and analysis of zeta-potential. The functionality of the nanovaccine was analyzed with the B3Z T-cell hybridoma assay, a colorimetric assay for ovalbumin-specific T-cell activation in-vitro[6]. Delivery to lymph nodes was performed by subcutaneous injections into the footpad of C57BL/6 mice. Nanoparticles were quantified in excised lymph nodes by Electron Paramagnetic Resonance (EPR) spectroscopy.
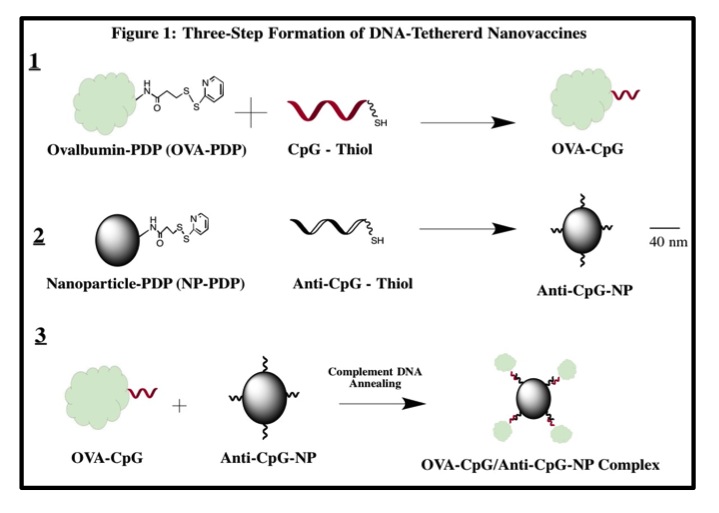
Results and Discussion: The successful formation of OVA-CpG conjugates was confirmed with anion exchange chromatography and spectrophotometric analysis. The elution of OVA-CpG from the High Q anion exchange column required significantly higher concentrations of NaCl as compared to free OVA (p = 0.001). Furthermore, the SYBR Gold assay specific for ssDNA revealed a significantly higher fluorescent signal for OVA-CpG conjugates as compared to free OVA (p = 0.03). Quantification of ssDNA and protein in Ova-CpG conjugates further confirmed that the conjugates could be formed with defined degree of CpG modification (~1, 2 and 3 mole CpG/mole OVA). Nanoparticle functionalization with anti-CpG and subsequent complexation with OVA-CpG to form OVA-CpG/anti-CpG-NP nanocomplexes was confirmed by agarose gel electrophoresis and zeta-potential (-1.8±0.5 mV, -27±1 mV and -40 ±2 mV for NP, anti-CpG-NP, and OVA-CpG/anti-CpG-NP, respectively, p<0.001). Functional analysis revealed that the OVA-CpG conjugates (1 mole CpG/mole OVA) and OVA-CpG/anti-CpG-NP nanocomplexes enhanced OVA-specific T cell activation by 2.5-fold (23 ± 1mU, p<0.001) and 4-fold (37±5 mU, p<0.001), respectively, as compared to free ovalbumin (9±1mU). Additionally, EPR quantification revealed delivery of approximately 25% of a subcutaneously injected dose of nanoparticles into axillary lymph nodes in mice 24 hours post-injection.
Conclusion: Results presented confirm successful preparation of OVA-CpG/anti-CpG-NP immunoactive DNA-tethered nanocomplexes. In addition, functional in vitro analysis and initial delivery results suggest that these nanocomplexes may combine two highly desirable cancer vaccine properties - enhanced immunological priming and enhanced lymph node delivery. The reported nanocomplexation method could pave the way to development of new potent cancer nanovaccines and warrants further investigation.
References:
[1] Emens, L. A. (2008). Cancer vaccines: on the threshold of success. Expert Opin Emerg Drugs, 13(2), 295-308. doi: 10.1517/14728214.13.2.295
[2] Copier, J., Dalgleish, A. G., Britten, et al. (2009). Improving the efficacy of cancer immunotherapy. Eur J Cancer, 45(8), 1424-1431. doi: 10.1016/j.ejca.2008.12.017
[3] Mehta, N. K., Moynihan, K. D., & Irvine, D. J. (2015). Engineering New Approaches to Cancer Vaccines. Cancer Immunol Res, 3(8), 836-843. doi: 10.1158/2326-6066.CIR-15-0112
[4] Bode, C., Zhao, G., Steinhagen, F., Kinjo, T., & Klinman, D. M. (2011). CpG DNA as a vaccine adjuvant. Expert Rev Vaccines, 10(4), 499-511. doi: 10.1586/erv.10.174
[5] Johnson, L., Pinder, S. E., & Douek, M. (2013). Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology, 62(3), 481-486. doi: 10.1111/his.12019
[6] Karttunen, J., Sanderson, S., & Shastri, N. (1992). Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A, 89(13), 6020-6024.